Article Archive
Article Archive
- Introduction of Cement Slurry System (Part 1)
- Introduction of Cement Slurry System (Part 2)
- Introduction of Cement Slurry System (Part 3)
- Introduction of Cement Slurry System (Part 4)
- High Temperature and High Pressure Cementing Technology
- Low Density Cementing Slurry Technology
- Anti Gas Channeling Cementing Technology
- Drag Reducing Agents (DRA) or Drag Reducers (DR)
- Nitrogen Surfactant Compound Huff and Puff Technology
- Oil Washing Technology for Increasing Production
2. Principle of crude oil pour point depressant
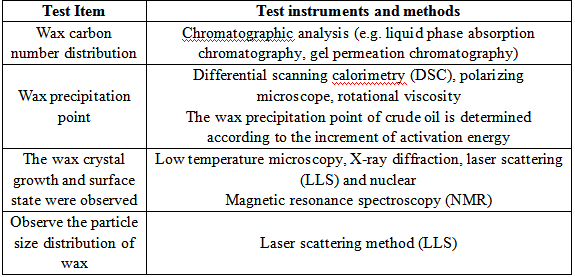
(1) Experimental instruments and methods for mechanism research
In the study of pour point reduction mechanism, it is very important to study the carbon number distribution of wax, determine the wax precipitation point and observe the growth of wax crystal before and after adding agent.
The adsorption theory holds that the crude oil pour point depressant precipitates at a temperature slightly lower than the cloud point of the crude oil and is adsorbed on the active center of the precipitated wax crystal nucleus to separate the wax crystals, so as to change the orientation of the wax crystals, make it difficult to form a three-dimensional mesh structure, and weaken the adhesion between the wax crystals Yenhneb also proposed that pour point depressant is not the solvent of crystal paraffin, nor will it reduce the content of paraffin in crude oil. It only changes the size, shape and structure of dispersed phase particles and adsorbs them on the surface of wax crystal to prevent the mutual proximity and adhesion of crystal particles, so as to change the rheological parameters of crude oil.
A. Adsorption theory
The adsorption theory holds that the crude oil pour point depressant precipitates at a temperature slightly lower than the cloud point of the crude oil and is adsorbed on the active center of the precipitated wax crystal nucleus to separate the wax crystals, so as to change the orientation of the wax crystals, make it difficult to form a three-dimensional mesh structure, and weaken the adhesion between the wax crystals Yenhneb also proposed that pour point depressant is not the solvent of crystal paraffin, nor will it reduce the content of paraffin in crude oil. It only changes the size, shape and structure of dispersed phase particles and adsorbs them on the surface of wax crystal to prevent the mutual proximity and adhesion of crystal particles, so as to change the rheological parameters of crude oil.
B. Nucleation theory
Nucleation theory is also known as crystallization center theory.
The theory holds that during the action of pour point depressant molecules, because the melting point of pour point depressant molecules is relatively higher than the crystallization temperature of wax in the oil, or the molecular weight of pour point depressant is larger than that of wax molecules, when the oil temperature decreases, pour point depressant molecules precipitate first than wax and become the center of wax crystal development, so that the small wax crystals formed in the cooling process of oil increase compared with those before the addition of agent, Thus, it is not easy to produce large wax mass and achieve the effect of reducing the freezing point.
C. Eutectic theory
According to the eutectic theory, the pour point depressant molecule has the same and different structural parts as the paraffin molecule, and the same part as the wax crystal is the hydrocarbon chain (non-polar group). In the process of wax crystallization and precipitation, it enters the lattice of the wax crystal and replaces the wax molecule (n-alkyl chain molecule) in the lattice to produce eutectic. Polar groups different from wax crystals hinder the further growth of wax crystals, slow down the growth in X and Y directions where wax crystals grow faster, and speed up the growth in Z direction where wax crystals grow slower. In this way, wax grows in an isotropic direction, reduces the ratio of surface area to volume, reduces the surface energy, and is not easy to form a network structure. In other words, the pour point depressant of crude oil crystallizes and precipitates together with wax below the cloud point temperature of crude oil, which destroys the crystallization behavior and orientation of wax and weakens the trend of continuous development of wax crystals.
D. Theory of improving the solubility of wax
According to the theory of improving the solubility of wax, pour point depressant is like surfactant. After adding pour point depressant, it increases the solubility of wax in oil, reduces the amount of wax precipitation, and increases the dispersion of wax. Due to the influence of surface charge after wax dispersion, wax crystals repel each other, which is not easy to coalesce to form three-dimensional network structure and reduce the freezing point. Crystallography also believes that if the additive improves the solubility of the solute, it will reduce the supersaturation of the solution, reduce the apparent growth rate and hinder the growth of the crystal. This theory is mainly used to explain the mechanism of polymers with surface active characteristics and dispersing wax.
Summary:
The role of pour point depressant in the process of crude oil additive modification is related to the type of pour point depressant and its own chemical structure. Moreover, at different stages of wax crystal growth, only one mechanism may play a major role, or several mechanisms may exist at the same time, which needs to be analyzed according to the specific situation.
Pour point reduction technology is a comprehensive technology based on many disciplines. Its development depends on the development of related disciplines such as petrochemical, crude oil rheology, thermodynamics, polymer synthesis, colloidal chemistry and oil transportation technology. The research on the mechanism of pour point depressant not only needs to adopt more reasonable experimental instruments and methods through comparison and study the compatibility law of wax before and after modification, but also needs to be combined with the design of pour point depressant molecules and the application of pour point depressant. The combination of theoretical research and practice has more guiding significance and practical value.




