Case Studies
Case Studies
- Construction of A New Organic-inorganic Composite Emulsion and Its Enhanced Mechanical Properties of Oil Well Cement(Part 1)
- Construction of A New Organic-inorganic Composite Emulsion and Its Enhanced Mechanical Properties of Oil Well Cement(Part 2)
- The Application Prospects of DeepSeek Large Model in Petroleum Engineering(Part 1)
- The Application Prospects of DeepSeek Large Model in Petroleum Engineering(Part 2)
- Development and Performance Evaluation of Efficient Asphalt Dispersant (Part 1)
- Development and Performance Evaluation of Efficient Asphalt Dispersant (Part 2)
- Development and Performance Evaluation of Efficient Asphalt Dispersant (Part 3)
- Research and Application Status of Drilling Fluid Plugging Materials (Part 1)
- Research and Application Status of Drilling Fluid Plugging Materials (Part 2)
- Research and Application Status of Drilling Fluid Plugging Materials (Part 3)

Abstract
In response to the problem of fluid loss agents in deep and ultra deep well drilling fluid systems being prone to degradation due to long-term high-temperature and high salinity effects. N-allyl aziridine (ALAI) was prepared by reacting aziridine with 3-chloropropene; Preparation of Tetraenol Crosslinking Agent Monomer (EAAD) by Reaction of ALAI with Ethylenediamine; EAAD undergoes an aqueous solution free radical copolymerization reaction with acrylamide (AM) and 2-acrylamido-2-methylpropanesulfonic acid (AMPS) to synthesize a micro crosslinked copolymer fluid loss additive (PAAT), Its structure and properties were characterized by 1H NMR, IR, and TG-DTG. The results show that PAAT can withstand high temperatures of 220 ℃ and maintain the filtration rate and rheological properties of drilling fluid under high temperature and high salinity conditions. The API filtration rate is less than 4.0mL, and the high-temperature and high-pressure filtration rate is less than 13.0mL.
With the depletion of shallow oil and gas resources, the exploration and development of oil and gas reservoirs gradually shift towards deep formations. Due to the high temperature of the lower wellbore and the long circulation time of the drilling fluid, the fluid loss agent in the drilling fluid system needs to adapt to high-temperature and high-pressure environments for a long time. Conventional linear macromolecular polymers are prone to main chain breakage or side chain degradation under high temperature, leading to weakened performance or complete failure. Introducing rigid large side groups and circular structures into linear polymer molecular structures can enhance the rigidity of molecular chains, increase molecular weight, inhibit the free movement of molecular segments, and thus ensure their structural stability in high-temperature environments. However, polymers with strong molecular chain rigidity and high molecular weight have poor water solubility, and cannot form a good compatibility with high-density, high solid-phase deep well drilling fluid systems. Introducing an appropriate amount of crosslinking agent in the preparation process of polymers to synthesize polymers with low crosslinking degree can limit the free movement trend of molecular chain segments near the crosslinking point, enhance molecular chain rigidity and mechanical and chemical stability, and meantime ensure that water solubility is not affected.
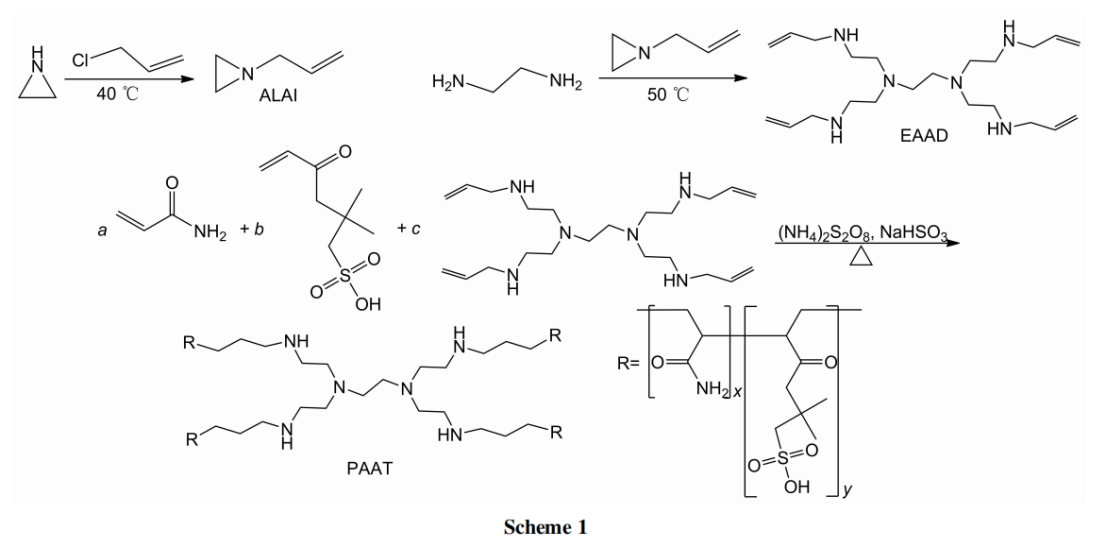
In this article, N-propenylaziridine (ALAI) was synthesized by the reaction of aziridine with 3-chloropropene; The reaction of ALAI with ethylenediamine yields a tetraalkenyl crosslinking agent monomer (EAAD); EAAD undergoes an aqueous solution free radical copolymerization reaction with acrylamide (AM) and 2-acrylamido-2-methylpropanesulfonic acid (AMPS) to synthesize a micro crosslinked copolymer fluid loss additive (PAAT, see Scheme 1), Its structure and properties were characterized by 1H NMR, IR, and TG-DTG.
1. Experimental Part
1.1 Instruments and Reagents
- BRGL-7 high-temperature rolling heating furnace;
- ZNN-D6S rotary viscometer;
- ZNS medium pressure filter tester;
- DFC-0705 high pressure high temperature filter tester;
- Bruker Advance III HD 400 MHz nuclear magnetic resonance instrument;
- WQF-520 infrared spectrometer (KBr tablet);
- STA-409 differential thermogravimetric synchronous analyzer.
- Bentonite, industrial product; All other reagents used are analytical pure.
1.2 Synthesis
(1) . Synthesis of ALAI
Dissolve 7.65g (0.1 mol) of 3-chloropropene in 150mL of anhydrous ethanol, raise the temperature to 40℃, slowly add dropwise 4.3g (0.1 mol) of ethanol (50mL) solution of aziridine , complete the dropwise addition, and reflux for 3 hours (TLC tracking). Remove ethanol by vacuum evaporation, and allow the residue to stand at 4℃ for 8 hours. Recrystallize with ethanol/acetone (4/1, V/V) to obtain light yellow solid ALAI; ¹H NMRδ:1.63(s,4H,CH2—N),3.14~3.24(m,2H,CH2—N),5.22(s,2H,CH2),5.88(s,1H,CH=C).
(2) . Synthesis of EAAD
Dissolve 6.0g (0.1 mol) of ethylenediamine in 150mL of anhydrous ethanol, raise the temperature to 50 ℃, slowly add dropwise 33.2g (0.4 mol) of ALAI ethanol solution, complete the drop, and reflux for 8 hours (TLC tracking). Remove ethanol by vacuum evaporation, and allow the residue to stand at 4℃ for 12 hours. Recrystallize with ethanol/acetone (4/1, V/V) to obtain a light yellow solid EAAD; ¹H NMRδ:2.37(s,4H,CH2—CH2),2.41(s,8H,N—CH2),2.51~2.54(m,8H,CH2—N),3.24(s,8H,N—CH2),3.36(s,4H,NH),5.23(s,8H,CH2),5.87(s,4H,CH=C).
(3) . Synthesis of PAAT
Dissolve AM, AMPS, and EAAD in deionized water (molar ratio 100/30/0.3, total monomer concentration 25 wt%), adjust to neutral with NaOH and pour into a three necked flask in N2 atmosphere. Stir and raise the temperature to 65℃, add 0.2 wt% ammonium persulfate and sodium bisulfite (molar ratio of 1/1) and react for 6 hours. Soak the crude product in acetone, let it stand for 24 hours, and then separate it with a cable extractor. Take the insoluble part and soak it in excess anhydrous ethanol. Let it stand for 24 hours, filter it, vacuum dry the filter cake, and crush it to obtain a white powder PAAT; ¹H NMRδ:1.32(s,CH),1.44(s,N—CH2),1.52(s,NH),1.55~1.64(m,CH2),2.36(s,CH2),2.39~2.41(t,CH2—CH2),2.43~2.52(m,N—CH2),3.03~3.21(m,CH),3.38(s,CH3),8.27(s,NH),8.37(s,SO3H).
1.3 Performance testing of PAAT
Aging treatment of PAAT aqueous solution was carried out using a rolling heating furnace at a temperature of 220 ℃ for 16 hours. The viscosity of the drilling fluid before and after aging treatment was tested using a six speed rotary viscometer.
2. Results and Discussion
2.1 Characterization
Figure 1 shows the IR spectra of EAAD and PAAT. According to the spectrum of EAAD in Figure 1, the absorption peak at 2952cm-1 is the stretching vibration peak of the C-H bond in the methylene group; The absorption peak at 1646cm-1 is the stretching vibration peak of the C=C bond; The absorption peak at 1472cm-1 is the bending vibration peak of the C-H bond in the methylene group; The absorption peak at 1360cm-1 is the stretching vibration peak of C-N in the tertiary amine group.
According to the spectrum of PAAT, the absorption peak at 3436cm-1 is the stretching vibration absorption peak of the N-H bond in AM; The absorption peak at 2956cm-1 is the stretching vibration peak of the C-H bond in the methylene group; The absorption peak at 1667cm-1 is the stretching vibration absorption peak of the C=O bond in AM and AMPS; The absorption peak at 1403cm-1 is the superposition peak of the in-plane bending vibration peak of methyl group and the C-N stretching vibration peak; The absorption peak at 1349cm-1 is the characteristic absorption peak of the N-CH3 bond in AM; The absorption peaks at 1191cm-1 and 1048cm-1 are symmetric vibrational peaks of sulfonic groups in AMPS; No absorption peak was observed at 1650-1620cm-1, indicating the absence of a C=C bond.
In summary, it can be seen that the molecular structure of PAAT is in line with expectations.
.png)
2.2 Thermal Performance
(1). TG-DTG
Figure 2 shows the TG-DTG curve of PAAT. From Figure 2, it can be seen that the TG curve of PAAT is mainly divided into three stages: the first stage is at 94~263℃, with a mass loss of 12.37%; From the DTG curve, it can be seen that the mass loss during this stage mainly occurs at 107℃, mainly due to the detachment of water molecules that combine with the carbonyl groups in the polymer molecular chain through hydrogen bonding; The second stage is from 263 to 376℃, during which the decrease in TG curve significantly increases, with a mass loss of 24.64%. However, the DTG curve shows a narrow and steep trough, with a corresponding temperature of 337℃ at the trough. The mass loss during this stage is mainly caused by the decomposition of - CONH2 in the side chains of polymer molecules, and the decomposition rate reaches its maximum at 337℃. The third stage is 376~471℃, during which the TG curve shows a significant downward trend, with a mass loss of 27.95%. The mass loss at this stage is mainly due to the high heating temperature, which causes the originally stable side chain SO3 groups in the polymer molecular structure to break with the C-N and C-C bonds in the main chain.
.png)
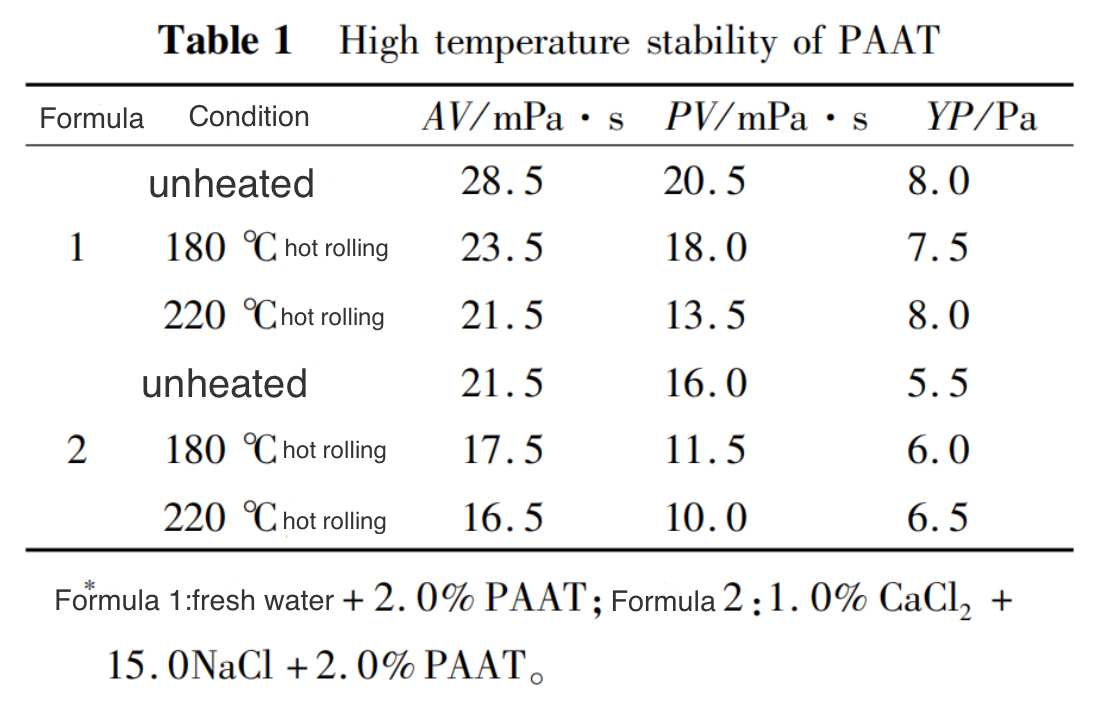
(2) . High Temperature Stability
Table 1 shows the high-temperature stability results of PAAT. From Table 1, it can be seen that PAAT has good water solubility. After high-temperature treatment, the viscosity of both fresh water and composite brine solutions of PAAT showed a slight decrease, while the dynamic shear force remained basically unchanged. Moreover, after 16 hours of hot rolling at 220℃, PAAT still maintained a certain viscosity and dynamic shear force in high salinity composite brine, indicating that the polymer can still maintain its polymer properties, which is beneficial for its application in deep well high-temperature and high salinity environments.