Case Studies
Case Studies
- Construction of A New Organic-inorganic Composite Emulsion and Its Enhanced Mechanical Properties of Oil Well Cement(Part 1)
- Construction of A New Organic-inorganic Composite Emulsion and Its Enhanced Mechanical Properties of Oil Well Cement(Part 2)
- The Application Prospects of DeepSeek Large Model in Petroleum Engineering(Part 1)
- The Application Prospects of DeepSeek Large Model in Petroleum Engineering(Part 2)
- Development and Performance Evaluation of Efficient Asphalt Dispersant (Part 1)
- Development and Performance Evaluation of Efficient Asphalt Dispersant (Part 2)
- Development and Performance Evaluation of Efficient Asphalt Dispersant (Part 3)
- Research and Application Status of Drilling Fluid Plugging Materials (Part 1)
- Research and Application Status of Drilling Fluid Plugging Materials (Part 2)
- Research and Application Status of Drilling Fluid Plugging Materials (Part 3)
2.3 Analysis of Mechanism of Action
(1) Zeta Potential
After the hydration of cement particles, the surface of the silicate phase is negatively charged, while the surface of the aluminate phase is positively charged. The electrostatic interaction between the two phases can cause the cement particles to coagulate.Zeta potential is an important parameter for understanding and simulating the interactions between particles and between polymers and particles in cement slurry.The Zeta potential of cement slurry with different dosages of CPC or DPC is shown in Figure 9.The Zeta potential of cement paste is -2.18mV. After adding CPC or DPC dispersant, the absolute value of the Zeta potential of the cement paste increases.The absolute value of Zeta potential of cement slurry containing CPC increases rapidly, while the absolute value of Zeta potential of cement slurry containing DPC increases slowly. This is because CPC only contains anions and can adsorb on the positive potential surface of cement particles, while DPC containing both anions and cations can adsorb on both the positive and negative potential surfaces of cement particles.
.png)
(2) Adsorption Layer Thickness
X-ray photoelectron spectroscopy (XPS) is a surface analysis technique that can obtain information on the types and relative contents of elements on the surface of materials.Figure 10 shows the XPS spectra of cement particles before and after adsorption of dispersants.Based on the difference in peak intensity, the relative elemental composition of the surface of blank cement and cement particles adsorbed with CPC or DPC was determined from the XPS spectrum, as shown in Table 3.In Table 3, after the adsorption of CPC or DPC on the surface of cement particles, the carbon content significantly increased.CPC or DPC does not contain calcium and silicon elements, resulting in a decrease in Ca and Si content, indicating that CPC or DPC has been adsorbed on the surface of cement particles.Comparing CPC and DPC, it can be found that the C content of CPC is slightly higher than that of DPC, indicating that CPC adsorbs more on the surface of cement particles than DPC;The contents of Ca, Si, and Al were significantly reduced compared to DPC, with a greater decrease in calcium ions than Si and Al, indicating that CPC has stronger calcium ion complexation ability than DPC. This also explains why CPC has higher dispersion efficiency than DPC and why CPC has strong retardation.
.png)
XPS can also be used to determine the thickness of the adsorption layer of specific substances. The principle is that some photoelectrons with characteristic energy decay exponentially when passing through the solid surface layer.Cement contains Si2p, while dispersants do not. After the dispersant adsorbs onto the surface of cement particles, the energy of Si2p photoelectrons will decay through the dispersant adsorption layer. Therefore, the thickness of the dispersant adsorption layer can be estimated based on the values before and after the weakening of Si2p photoelectron intensity, and the adsorption of dispersants on the surface of cement particles can be explored.Figure 11 shows the XPS spectra of Si2p before and after the adsorption of different dispersants on cement particles. The measured spectral line area is the photoelectron intensity I of Si2p.
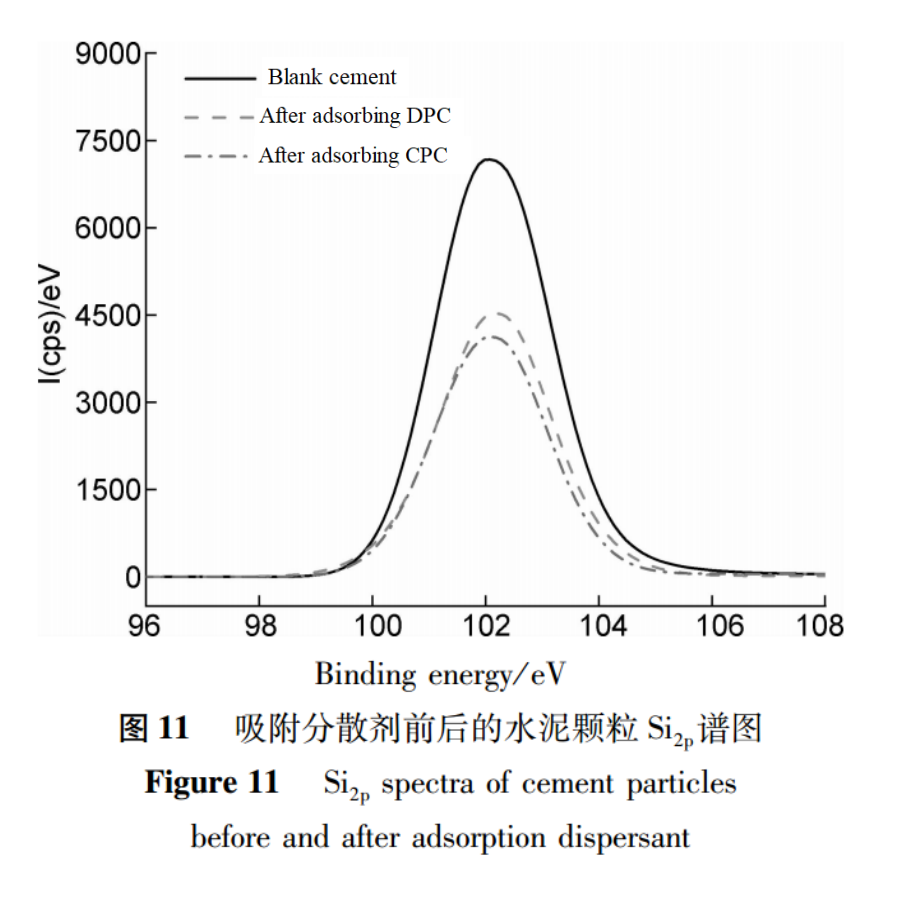
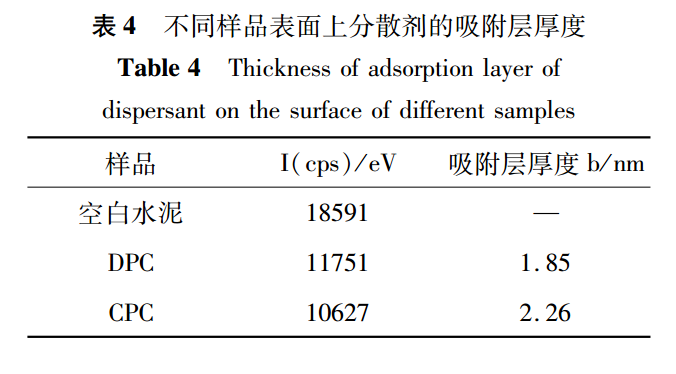
The adsorption layer thickness of CPC and DPC on the surface of cement particles was calculated using literature methods, as shown in Table 4.In Table 4, the adsorption layer thickness generated by DPC on the surface of cement particles is 1.85nm, which is smaller than the adsorption layer thickness generated by CPC on the surface of cement particles, which is 2.26nm. This further explains why the dispersion efficiency of DPC is slightly lower than that of CPC.For polycarboxylate dispersants, a thicker adsorption layer indicates better side chain extension and greater steric hindrance, which is more conducive to the dispersion of cement particles.
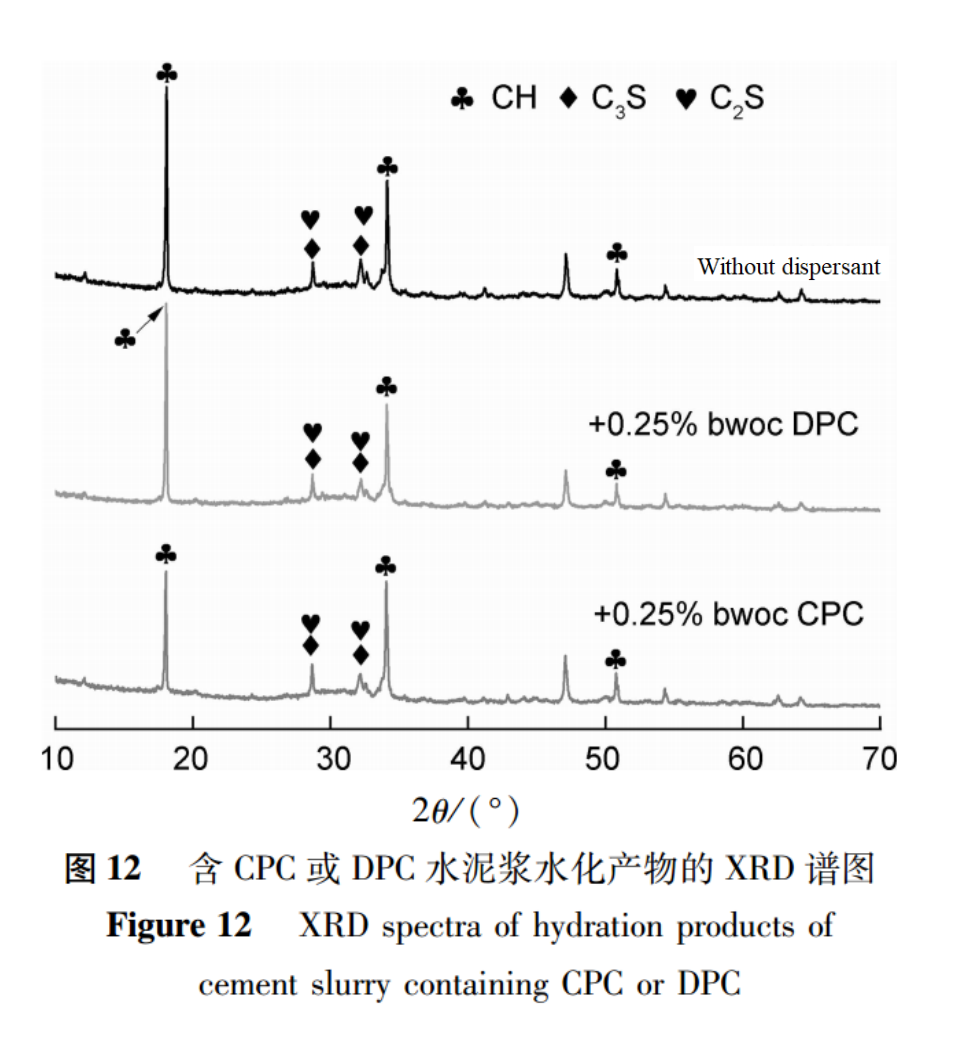
(3) Phase Analysis of Hydration Products
The X-ray diffraction (XRD) spectra of hydration products without dispersants and cement slurry containing 0.25% CPC or DPC cured at 85℃ for 24 hours are shown in Figure 12.The types of hydration products of all cement stones are the same, mainly including calcium hydroxide (CH), hydrated calcium silicate (C-S-H), as well as unreacted tricalcium silicate (C3S) and dicalcium silicate (C2S),CH has three strong peaks at 2θ={18.01, 34.10, 50.08}, C-S-H shows a wide hump at 2θ=[25~35], and C3S and C2S have two diffraction peaks at 2θ≈28.78 and 32.27.The intensity of diffraction peaks can to some extent reflect the content of corresponding substances in the sample. Comparing the diffraction peaks of the 24-hour hydration product CH at 2θ=18.01 among the three, it can be found that the CH peak intensity of DPC containing cement slurry is equivalent to that of cement slurry without dispersant.The peak strength of CH in cement slurry containing CPC is significantly lower than that in cement slurry without dispersant. This is because CPC delays the hydration rate of cement clinker, resulting in a relatively low content of hydration product CH, which explains why CPC prolongs the thickening time of cement slurry and leads to a decrease in early strength of cement stone.
3.Conclusion
In order to solve the environmental problem of "de sulfonation" of existing sulfonated aldehyde ketone condensate dispersants, conventional anionic polycarboxylate (CPC) and zwitterionic polycarboxylate (DPC) dispersants were synthesized, and the application performance and mechanism of CPC and DPC in cementing slurry were compared.DPC is synthesized by replacing some carboxylic acids in CPC with quaternary ammonium cations. Both DPC and CPC have good dispersibility, but CPC has strong calcium ion complexation ability and a strong retarding effect, resulting in a significant extension of thickening time and a decrease in the content of calcium hydroxide, the main hydration product, which is not conducive to the early strength development of mudstone.The introduction of quaternary ammonium cations in DPC significantly reduces the retarding side effects of CPC, and has the advantages of high dispersion and low retarding. It has broad application prospects in cementing slurry.



