Case Studies
Case Studies
- Construction of A New Organic-inorganic Composite Emulsion and Its Enhanced Mechanical Properties of Oil Well Cement(Part 1)
- Construction of A New Organic-inorganic Composite Emulsion and Its Enhanced Mechanical Properties of Oil Well Cement(Part 2)
- The Application Prospects of DeepSeek Large Model in Petroleum Engineering(Part 1)
- The Application Prospects of DeepSeek Large Model in Petroleum Engineering(Part 2)
- Development and Performance Evaluation of Efficient Asphalt Dispersant (Part 1)
- Development and Performance Evaluation of Efficient Asphalt Dispersant (Part 2)
- Development and Performance Evaluation of Efficient Asphalt Dispersant (Part 3)
- Research and Application Status of Drilling Fluid Plugging Materials (Part 1)
- Research and Application Status of Drilling Fluid Plugging Materials (Part 2)
- Research and Application Status of Drilling Fluid Plugging Materials (Part 3)
2.2.2 Effect of Total Monomer Dosage
Figure 6 shows the effect of total monomer dosage on the filtration performance of PAAAA. From Figure 6, it can be seen that as the total amount of monomers increases, both FLAPI and FLHTHP first decrease and then slowly increase. When the total amount is 20% (w), FLAPI and FLHTHP are the lowest. This is because when the total amount of monomers is small, the relative molecular weight of PAAAA is smaller and the filtration loss is higher. As the total amount of monomers increases, the probability of free radical collisions increases, and the relative molecular weight of PAAAA also gradually increases, resulting in a decrease in filtration loss. However, as the total amount of monomers continues to increase, the concentration of free radicals increases, chain transfer and termination rates increase, and the relative molecular weight of PAAAA decreases. Therefore, the appropriate total dosage of monomers is 20% (w).
.png)
2.2.3 Effect of Initiator Dosage
Figure 7 shows the filtration loss reduction performance of PAAAA in freshwater based slurry under different initiator dosages. From Figure 7, it can be seen that with the increase of initiators, FLHTHP first decreases and then increases. When the dosage of initiator is 0.16% (w), FLAPI and FLHTHP are the lowest. This is because when the amount of initiator added is small, the initiation efficiency is lower, and the relative molecular weight of PAAAA is lower; When the amount of initiator is too high, the concentration of free radicals increases, the polymerization rate accelerates, resulting in a decrease in the relative molecular weight of PAAAA and an increase in filtration loss. Therefore, it is more appropriate to choose an initiator dosage of 0.16% (w) of the total mass of the polymerization monomer.
.png)
2.2.4 Effect of Reaction Temperature
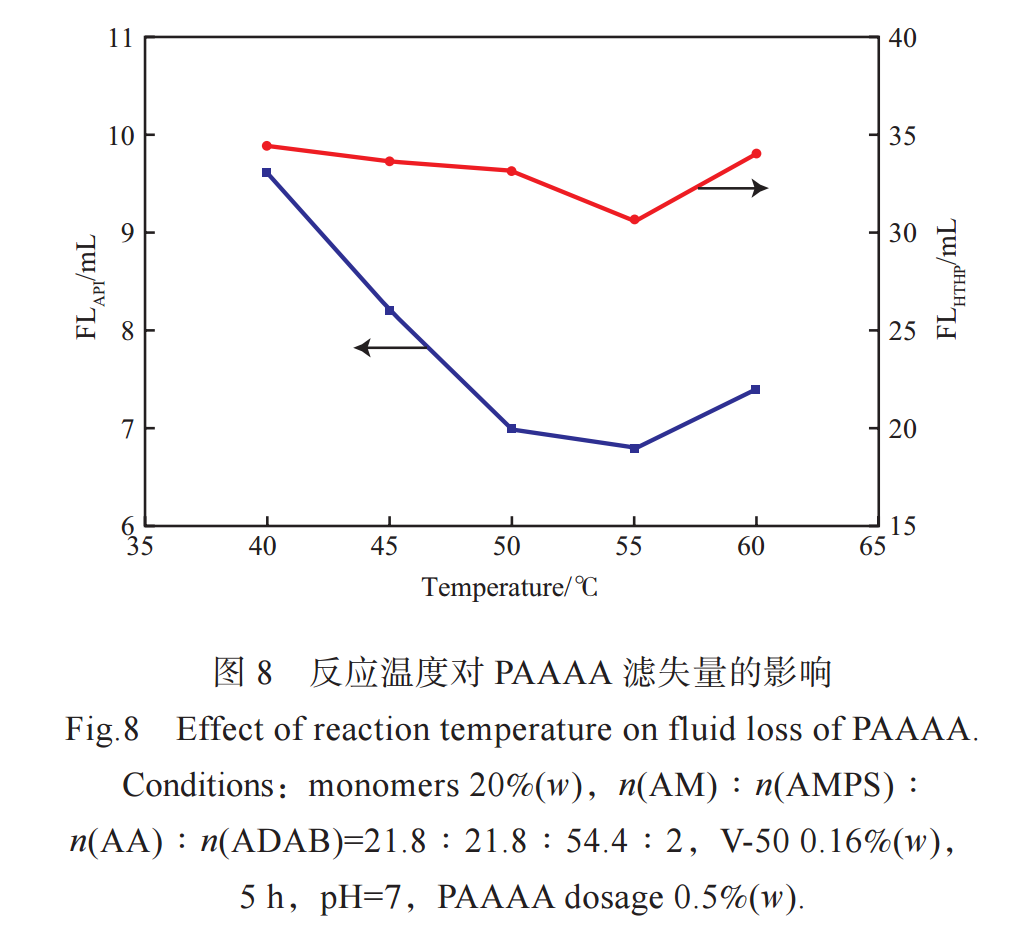
The effect of reaction temperature on the filtration loss of PAAAA in freshwater slurry is shown in Figure 8. From Figure 8, it can be seen that as the reaction temperature increases, FLAPI and FLHTHP first decrease and then increase. When the temperature is 55℃, FLAPI and FLHTHP are the lowest. When the temperature is low, the decomposition rate of the initiator is low, the reaction rate is low, the relative molecular weight of the generated PAAAA is small, and the filtration loss is large. But when the temperature is too high, the decomposition rate of the initiator increases, and a large number of free radicals are generated in a short period of time, leading to an increase in chain transfer and termination rate, and releasing a large amount of heat. Although the reaction at this time is intense, the relative molecular weight of the synthesized PAAAA is not high. Therefore, choosing a reaction temperature of 55 ℃ is more appropriate.
2.2.5 Effect of Reaction Time
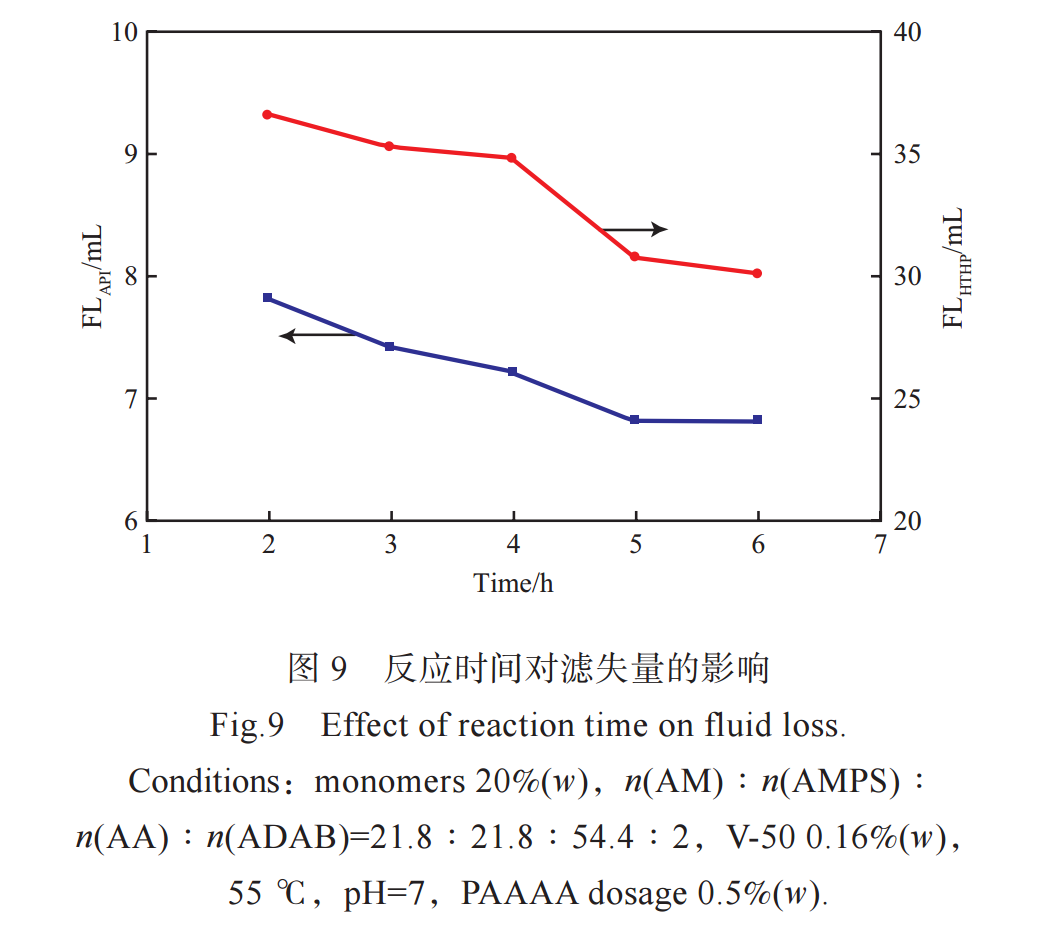
Figure 9 shows the effect of reaction time on the filtration performance of PAAAA. From Figure 9, it can be seen that when the reaction time is short and the reaction is incomplete, the relative molecular weight of the generated PAAAA is low and the filtration loss is large. As the reaction time prolongs, the relative molecular weight of PAAAA increases and the filtration rate gradually decreases. However, after the reaction exceeds 5 hours, the filtration rate of synthesized PAAAA no longer significantly decreases, indicating that the polymerization reaction is basically complete and the relative molecular weight of PAAAA has little change. Therefore, the reaction time was chosen as 5 hours.
2.3 Factors Affecting the Filtration Performance of PAAAA
2.3.1 Effect of dosage
The effect of PAAAA dosage on its filtration performance in freshwater slurry is shown in Table 1. From Table 1, it can be seen that with the increase of PAAAA content, FLAPI and FLHTHP gradually decrease. When the PAAAA content is 0.5% (w), FLAPI and FLHTHP are 8.3 and 31.6 mL, respectively. This indicates that in freshwater based slurries, PAAAA still has good filtration loss reduction performance even in small amounts.
.png)
2.3.2 Salt Resistance
The filtration performance of PAAAA in saturated saline slurry is shown in Table 2. From Table 2, it can be seen that with the increase of PAAAA content, both FLAPI and FLHTHP show a continuous decreasing trend. When the PAAAA content is 1.5% (w), FLAPI and FLHTHP are 3.6 mL and 16.0mL, respectively. When the content exceeds 1.5% (w), the change in filtration loss is not significant, indicating that the appropriate PAAAA content is 1.5% (w) in saturated saline base slurry.
.png)
2.3.3 Temperature Resistance Performance
Table 3 shows the effect of aging temperature on the filtration performance of PAAAA in freshwater slurry. From Table 3, it can be seen that with the increase of aging temperature, FLAPI shows an increasing trend, while FLHTHP does not show much change before 200℃, but increases after exceeding 200℃. After aging at 200℃ for 16 hours, FLAPI and FLHTHP were 9.0 mL and 26.8 mL respectively; After aging at 220℃ for 16 hours, FLAPI and FLHTHP were 13.8 mL and 36.8 mL, respectively. It indicates that PAAAA has good filtration loss reduction performance, can withstand high temperatures of 200℃, and still has a certain effect at 220℃.
.png)
2.4 Zeta Potential and Particle Size Analysis
The effect of ADAB dosage on the Zeta potential and average particle size of PAAAA in freshwater slurry is shown in Table 4. From Table 4, it can be seen that as the amount of ADAB increases, the Zeta potential of the base slurry first decreases and then increases. This is because ADAB is a cationic monomer, and when its dosage is small, the side chain part enters the interlayer of montmorillonite through adsorption, and the polymer main chain adsorbs on the surface. Moreover, due to the presence of a large number of hydration groups, the negative charge on the clay surface increases and the Zeta potential decreases; As the amount of ADAB increases, some of the cations do not enter the interlayer of the montmorillonite layer. Due to the chain length, they will adsorb with the anionic groups on the main chain, resulting in an increase in Zeta potential. The trend of average particle size and Zeta potential is basically consistent. When the surface electronegativity of clay increases, the mutual repulsion between particles also increases, making it difficult for clay particles to aggregate, resulting in smaller particle sizes. However, when the surface electronegativity of clay decreases, the trend of mutual aggregation between particles increases, making clay particles larger and the average particle size increases.
.png)
3. Conclusion
1) The suitable conditions for synthesizing fluid loss agent PAAAA are n (AM): n (AMPS): n (AA): n (ABAD)=21.8:21.8:54.4:2, the total amount of monomers is 20% (w), the amount of initiator is 0.16% (w), the reaction temperature is 55℃, and the reaction time is 5 hours.
2) The prepared PAAAA has good filtration loss reduction performance and temperature and salt resistance. The FLAPI and FLHTHP of the saturated saline based slurry with a PAAAA content of 1.5% (w) are 3.6 mL and 16.0 mL, respectively; After aging at 200℃ for 16 hours, the FLAPI and FLHTHP were 9.0mL and 26.8 mL, respectively. After aging at 220℃, they still showed certain filtration loss reduction performance.
3) The addition of a small amount of long chain quaternary ammonium salt monomer ADAB results in significant electronegativity of clay particles and can maintain particle stability.



