Case Studies
Case Studies
- Construction of A New Organic-inorganic Composite Emulsion and Its Enhanced Mechanical Properties of Oil Well Cement(Part 1)
- Construction of A New Organic-inorganic Composite Emulsion and Its Enhanced Mechanical Properties of Oil Well Cement(Part 2)
- The Application Prospects of DeepSeek Large Model in Petroleum Engineering(Part 1)
- The Application Prospects of DeepSeek Large Model in Petroleum Engineering(Part 2)
- Development and Performance Evaluation of Efficient Asphalt Dispersant (Part 1)
- Development and Performance Evaluation of Efficient Asphalt Dispersant (Part 2)
- Development and Performance Evaluation of Efficient Asphalt Dispersant (Part 3)
- Research and Application Status of Drilling Fluid Plugging Materials (Part 1)
- Research and Application Status of Drilling Fluid Plugging Materials (Part 2)
- Research and Application Status of Drilling Fluid Plugging Materials (Part 3)
2.1.4 Optimization of reaction time
The reaction time affects the monomer conversion rate and the molecular weight of the filtrate reducer, thereby affecting the filtrate reduction effect. Constant monomer ratio n(AM):n(APEG-1000):n(SSS):n(DMAAC-18)=110:3:10:1,initiator dosage 0.4% (w), total monomer content 30% (w), reaction temperature 60℃, reaction pH=7, and change the reaction time to synthesize a series of fluid loss agent products. Add 1.25% (w) of fluid loss agent to the freshwater base slurry and measure the API filtration rate. The results are shown in Figure 3.
From Figure 3, it can be seen that as the reaction time increases, the filtrate loss reduction effect of the obtained filtrate reducer continuously improves. When the reaction time is 4 hours, the optimal filtration reduction effect is achieved, with an API filtration rate of 7.4 mL. Because when the polymerization time is short, the monomer conversion rate is low and the reaction is not sufficient; When the reaction time is 4 hours, the monomer conversion rate is close to complete, and the obtained filtrate reducer has the best filtrate reduction effect; When the reaction time exceeds 4 hours, the reaction has stopped and the molecular weight of the polymer no longer changes, so it has no effect on the filtration reduction effect. Therefore, the reaction time was determined to be 4 hours.
.png)
2.1.5 Optimization of total monomer content
The total monomer content affects the collision frequency of polymerization, resulting in changes in polymer molecular weight. Constant monomer ratio n(AM):n(APEG-1000):n(SSS):n(DMAAC-18)=110:3:10:1, initiator dosage 0.4% (w), reaction temperature 60℃, reaction time 4 hours, reaction pH=7, changing the total monomer content to synthesize a series of fluid loss agent products. Add 1.25% (w) of fluid loss agent to the freshwater base slurry and measure the API filtration rate. The results are shown in Figure 4.
From Figure 4, it can be seen that when the total monomer content is 30% (w), the API filtration rate of the obtained filtrate reducer is 7.4 mL, and the filtrate reduction effect is the best. When the total monomer content is low, the probability of monomer collision is low and the molecular weight of the polymer is small; When the monomer content is too high, it will lead to an increase in the viscosity of the polymerization reaction system, blocking free radicals at the active chain end, accelerating chain termination, and reducing the molecular weight of the polymer. Therefore, the total monomer content was determined to be 30% (w).
.png)
2.1.6 Optimization of reaction pH
The reaction pH affects the reaction rate by controlling the decomposition rate of the initiator, thereby affecting the polymer filtration reduction effect. Constant monomer ratio n (AM): n (APEG-1000): n (SSS): n (DMAAC-18)=110:3:1:1, initiator dosage 0.4% (w), monomer content 30% (w), reaction temperature 60 ℃, reaction time 4 hours, and changing reaction pH to synthesize a series of fluid loss agent products. Add 1.25% (w) of fluid loss agent to the freshwater base slurry and measure the API filtration rate. The results are shown in Figure 5.
As shown in Figure 5, as the reaction pH increases, the API filtration rate of the obtained filtrate reducer first decreases and then increases. When the reaction pH is 8, the filtrate reduction effect is the best, with an API filtration rate of 7.3 mL. This is because the initiator can only exert its maximum effect within a certain pH range. When the pH is low, the system becomes acidic, the initiator slowly decomposes, the monomer conversion rate is low, and the polymer molecular weight is low; When the pH is high, the system is alkaline, and the initiator rapidly decomposes, producing a large number of free radicals, accelerating the reaction rate, premature chain termination and transfer, and low polymer molecular weight, resulting in an increase in filtration loss. Therefore, it was determined that the reaction pH was 8.
.png)
2.2 Structural characterization of high-temperature resistant drilling fluid loss reducing agent
2.2.1 FTIR characterization results
Figure 6 shows the FTIR spectrum of the synthesized polymer under optimal conditions after purification and drying.
From Figure 6, it can be seen that the absorption peak at 3546 cm-1 is the stretching vibration of the N-H bond on the amide group; The absorption peaks at 2925 cm-1 and 2865 cm-1 are the stretching vibrations of C-H bonds on methyl and methylene groups, respectively; The absorption peak at 1679 cm-1 is the stretching vibration of the C=O bond on the amide group; The absorption peak at 1452 cm-1 is the stretching vibration of the double bonds on the benzene ring skeleton; The absorption peak at 1189 cm-1 is the stretching vibration of the C-O-C ether bond on the polyoxyethylene ether; The absorption peak at 1110 cm-1 is the asymmetric stretching vibration of the S=O bond on the sulfonic acid group; The absorption peak at 1035 cm-1 is the symmetric stretching vibration of the S=O bond on the sulfonic acid group. It can be seen that four monomers have successfully synthesized fluid loss agents through polymerization reaction, meeting the design requirements.
.png)
2.2.2 Thermal stability characterization results
Figure 7 shows the TG-DTG curve of the fluid loss additive.
From Figure 7, it can be seen that the thermal decomposition of fluid loss agents is mainly divided into three stages: weight loss caused by hydrophilic groups in the polymer molecular structure at 40-235℃; 235~332℃ refers to the thermal decomposition of amide groups in polymers, ether bond breakage, and weight loss caused by polymer melting under heat; A large thermal weight loss plateau appeared at 332-499℃, with a fast and then slow rate of thermal weight loss. At this stage, the sulfonic acid groups within the polymer molecules began to decompose, and the C-C bond began to break, resulting in a sudden decrease in the weight loss curve. This indicates that the thermal decomposition temperature of the polymer is about 332℃, indicating that the synthesized fluid loss additive has good thermal stability.
.png)
2.3 Performance evaluation of high-temperature resistant drilling fluid loss agent
2.3.1 Effect of Fluid Loss Additive Dosage on Fluid Denaturation and Filtration Performance
The amount of fluid loss additive can have a significant impact on the performance of drilling fluid. Different amounts of fluid loss additive are added to the freshwater base mud, and the rheological parameters and API filtration rate before and after aging at 220 ℃ are measured. The results are shown in Table 2.
From Table 2, it can be seen that as the amount of fluid loss reducer increases, the viscosity increases and the API filtration rate decreases.When the dosage of fluid loss agent is 1.25% (w), the API filtration rate before aging decreases from 32.0 mL to 7.3 mL, and the API filtration rate after aging decreases from 137.0 mL to 13.2 mL; When the dosage of fluid loss additive is increased to 1.50% (w), the API filtration rate before aging decreases to 7.0 mL, and the API filtration rate after aging decreases to 13.0 mL. The decrease in filtration rate is reduced, and considering factors such as cost, the optimal dosage of fluid loss additive is 1.25% (w).
This result indicates that the fluid loss additive has positive rheological regulation and controllable filtration performance. Due to the adsorption of amide groups and polyoxyethylene ethers on the surface of clay, the adsorption groups in the fluid loss agent molecules prevent the aggregation of clay particles. The long chains of polyoxyethylene ethers are easy to enter the interlayer of bentonite, enhancing the dispersibility of clay. The strong hydration group sulfonic acid group forms a hydration film on the surface of clay, preventing clay condensation.
.png)
AV:apparent viscosity;PV:plastic viscosity.
2.3.2 Evaluation of temperature resistance performance
High temperature degradation is the main reason for the failure of fluid loss additives, therefore temperature is an important factor affecting the performance of drilling fluids. Add 1.25% (w) fluid loss reducing agent to the freshwater base slurry, stir at high speed and mix evenly. Measure the rheological parameters and API filtration rate after aging at different high temperatures for 16 hours. The results are shown in Table 3.
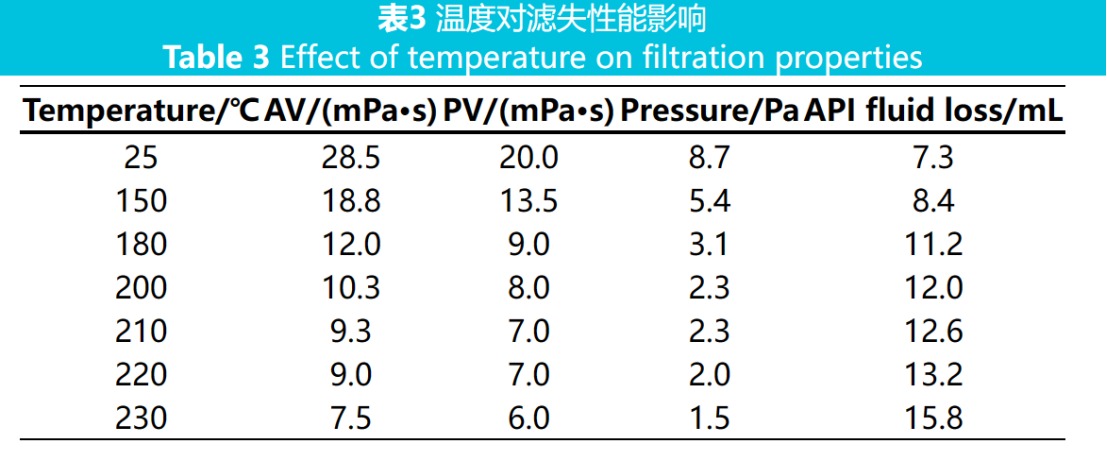
From Table 3, it can be seen that as the temperature increases from 150 ℃ to 230 ℃, the viscosity of the drilling fluid gradually decreases and the API filtration rate slightly increases. This is because ultra-high temperatures can lead to the thermal degradation of molecular chains, and the hydrolysis trend of the adsorption group amide group on the side chain will also increase, resulting in a macroscopic decrease in drilling fluid viscosity. When the aging temperature is 220 ℃, the API filtration rate is 13.2 mL, indicating that the filtrate reducer has good thermal stability and filtrate reduction performance. Because the sulfonic acid groups in the fluid loss agent molecules have strong hydration ability and have a high-temperature resistant rigid benzene ring, the clay particles can still maintain the thickness of the hydration film at high temperatures; The cations on the long side chain of the fluid loss agent undergo multi-point adsorption with negatively charged clay, making it difficult for clay particles to desorb under high temperature. The grid structure formed by the long chain under high temperature can also enclose a layer of free water, reducing the amount of free water penetration.
2.3.3 Compatibility Study of Fluid Loss Additives
To study the performance impact of fluid loss additives in water-based drilling fluid system formulations, fluid loss additives were combined with other additives to form drilling fluid systems 1# and 2#, and their compatibility with other additives was investigated. The results are shown in Table 4.
System 1# formula: fresh water base slurry+1.25% (w) fluid loss additive+5% (w) SMP fluid loss additive+0.5% (w) FA367 coating agent; System 2# formula: fresh water base slurry+1.25% (w) PAC-LV fluid loss additive+5% (w) SMP fluid loss additive+0.5% (w) FA367 coating agent.
From Table 4, it can be seen that the viscosity of drilling fluid system 1# and 2# is higher than that of system 2# before and after aging, indicating that the rheological properties of drilling fluid system 1# are more stable.
In systems 1 # and 2 # mixed with SMP fluid loss additive, after replacing PAC-LV fluid loss additive with this fluid loss additive, the API filtration rate before aging decreased from 5.6 mL to 4.4 mL, the API filtration rate after aging at 220 ℃ decreased from 13.8 mL to 5.2 mL, and the HTHP filtration rate after aging at 220 ℃ decreased from 35.2 mL to 13.6 mL. This indicates that this fluid loss additive has better compatibility with SMP fluid loss additive and has excellent performance in high-temperature drilling. It can be effectively applied in polysulfonic acid drilling fluid systems.

HTHP:high temperature and high pressure.



