Case Studies
Case Studies
- Construction of A New Organic-inorganic Composite Emulsion and Its Enhanced Mechanical Properties of Oil Well Cement(Part 1)
- Construction of A New Organic-inorganic Composite Emulsion and Its Enhanced Mechanical Properties of Oil Well Cement(Part 2)
- The Application Prospects of DeepSeek Large Model in Petroleum Engineering(Part 1)
- The Application Prospects of DeepSeek Large Model in Petroleum Engineering(Part 2)
- Development and Performance Evaluation of Efficient Asphalt Dispersant (Part 1)
- Development and Performance Evaluation of Efficient Asphalt Dispersant (Part 2)
- Development and Performance Evaluation of Efficient Asphalt Dispersant (Part 3)
- Research and Application Status of Drilling Fluid Plugging Materials (Part 1)
- Research and Application Status of Drilling Fluid Plugging Materials (Part 2)
- Research and Application Status of Drilling Fluid Plugging Materials (Part 3)
2.1.1.3 Dosage of AA
AA contains carboxylic acid groups with good hydrophilicity, which can increase the hydration effect of polymers on clay particles, thereby improving the slurry making ability of polymers in drilling fluids. Moreover, carboxylic acid groups can effectively inhibit the hydrolysis of amide groups. Fixed m (AM): m (AMPS): m (SSS): m (DMDAAC)=30:40:8:7, the filtration loss reduction effect of PAASDA in freshwater slurry was investigated under different AA dosages. The results are shown in Figure 2. From Figure 2, it can be seen that with the increase of AA dosage, the filtration loss of PAASDA in freshwater slurry first decreases and then increases. This is because the carboxylic acid groups in AA can improve the mud making performance of drilling fluid, resulting in a decrease in polymer filtration loss. However, when the dosage is too large, the filtration loss actually increases. So the appropriate amount of AA is determined to be 15% (w).
.png)
2.1.2 Dosage of Initiator
The effect of initiator dosage on the filtration performance of PAASDA in freshwater slurry is shown in Figure 3. From Figure 3, it can be seen that when the dosage of initiator is 0.6% (w), PAASDA has the best filtration loss reduction effect in freshwater slurry, and with the increase of initiator system dosage, FLAPI first decreases and then increases.
.png)
2.2 Structure and Properties of Polymers
2.2.1 Structural Characterization
The FTIR and 1H NMR spectra of PAASDA synthesized under optimal conditions are shown in Figures 4-5. From Figure 4, it can be seen that the stretching vibration absorption peak of the N-H bond is located at 3467 cm-1; The stretching vibration absorption peak of the C=O bond is located at 1674cm-1; The stretching vibration absorption peak of C-N bond is at 1411cm-1; indicating that the synthesized polymer contains amide groups. The characteristic absorption peaks of the benzene ring are located at 1600, 1500, 1450, and 1542 cm-1, while the absorption peaks of sulfonic acid groups are located at 1043, 1179, 662, and 545 cm-1, indicating that the synthesized polymer contains both benzene and sulfonic acid groups. The absorption peak at 1470 and 2919 cm-1 indicates the presence of quaternary ammonium salt groups and two methyl groups, which is consistent with the pentacyclic quaternary ammonium salt ring formed by DMDAAC polymerization. The characteristic peak of sodium carboxylate is at 1226 and 1005cm-1.
.png)
.png)
From Figure 5, it can be seen that the chemical shift at δ=7.3,7.6 corresponds to the benzene ring hydrogen in SSS; The chemical shift at δ=6.0 corresponds to the hydrogen of -NH2 in AM; At δ=3.8, 1.4 corresponds to the methylene group connected to the sulfonic acid group in AMPS and the hydrogen in the two methyl groups; At δ=3.6, 3.1 corresponds to the hydrogen on the two methyl groups and two methylene groups connected to N+ in DMDAAC; δ=2.4, 2.2, 1.8, and 1.1 respectively represent the hydrogen of SSS, AM, AMPS, and DMDAAC on the carbon skeleton.
The DTA-TG curve of PAASDA is shown in Figure 6. From Figure 6, it can be seen that at 178-215℃, the free water in the polymer is basically evaporated by heating, and -COO- begins to decompose, and the decomposition rate gradually increases with the increase of temperature; At 291-373℃, the mass loss almost linearly decreases, indicating that at this stage, the main and side chains of the polymer begin to break, the copolymer begins to thermally decompose, and weight loss increases rapidly. Before 291℃, the mass retention rate of the polymer was above 63%, indicating that PAASDA has strong thermal stability and excellent temperature resistance.
.png)
2.2.3 Saltwater based Slurry
2.2.3.1 Effect of Polymer Dosage on the Performance of Base Slurry
Add PAASDA fluid loss agent to the saline base slurry, and the results are shown in Figure 7. From Figure 7, it can be seen that as the amount of PAASDA increases, FLAPI decreases. When the amount of PAASDA is 2.0% (w), FLAPI is 6.4mL. After aging at 150℃, FLAPI is 8.0mL, indicating that the polymer has a significant filtration loss reduction effect. This is because PAASDA contains sulfonic acid groups and has good salt resistance, so it can be well applied in saltwater based mud drilling fluids.
.png)
2.2.3.2 The Influence of Temperature on the Performance of Base Slurry
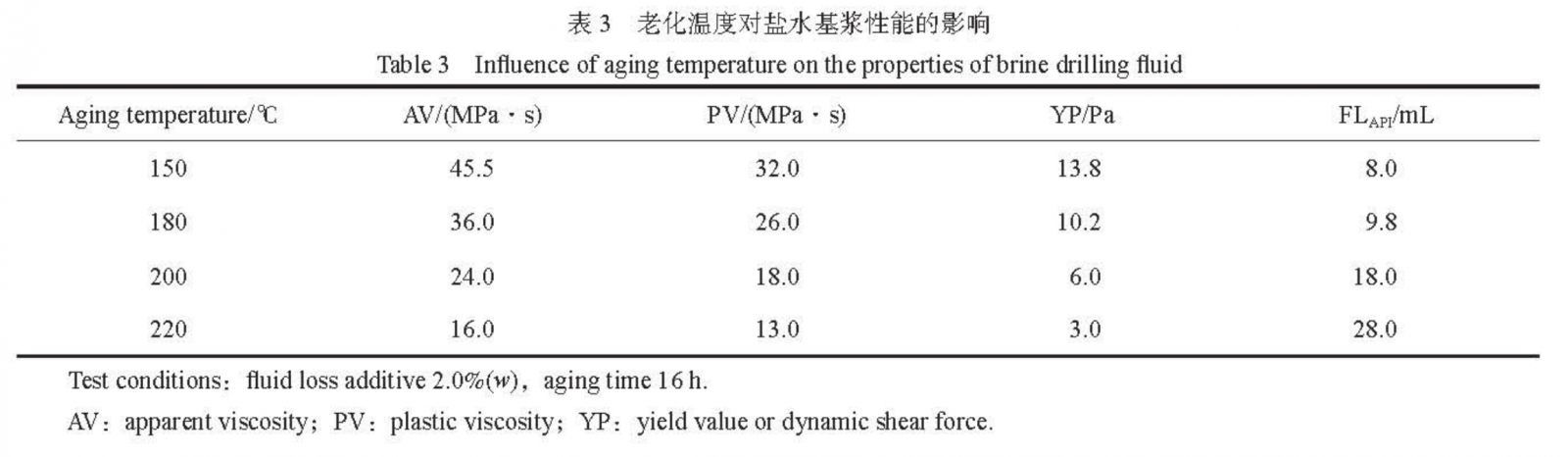
The effect of aging temperature on the performance of saline based slurry is shown in Table 3. From Table 3, it can be seen that after aging at 180℃, the FLAPI is 9.8mL, which is lower than 10.0mL; After aging at 200℃, although FLAPI was 18.0mL, the rheological parameters did not decrease significantly, indicating that the drilling fluid still has the ability to carry sand. PAASDA still exhibits good performance in saltwater based slurries at 180℃, indicating that the PAASDA fluid loss agent can resist 180℃.
2.2.4 Comparison of Performance with Commonly used Sulfonation Fluid Loss Agents in Oil Fields
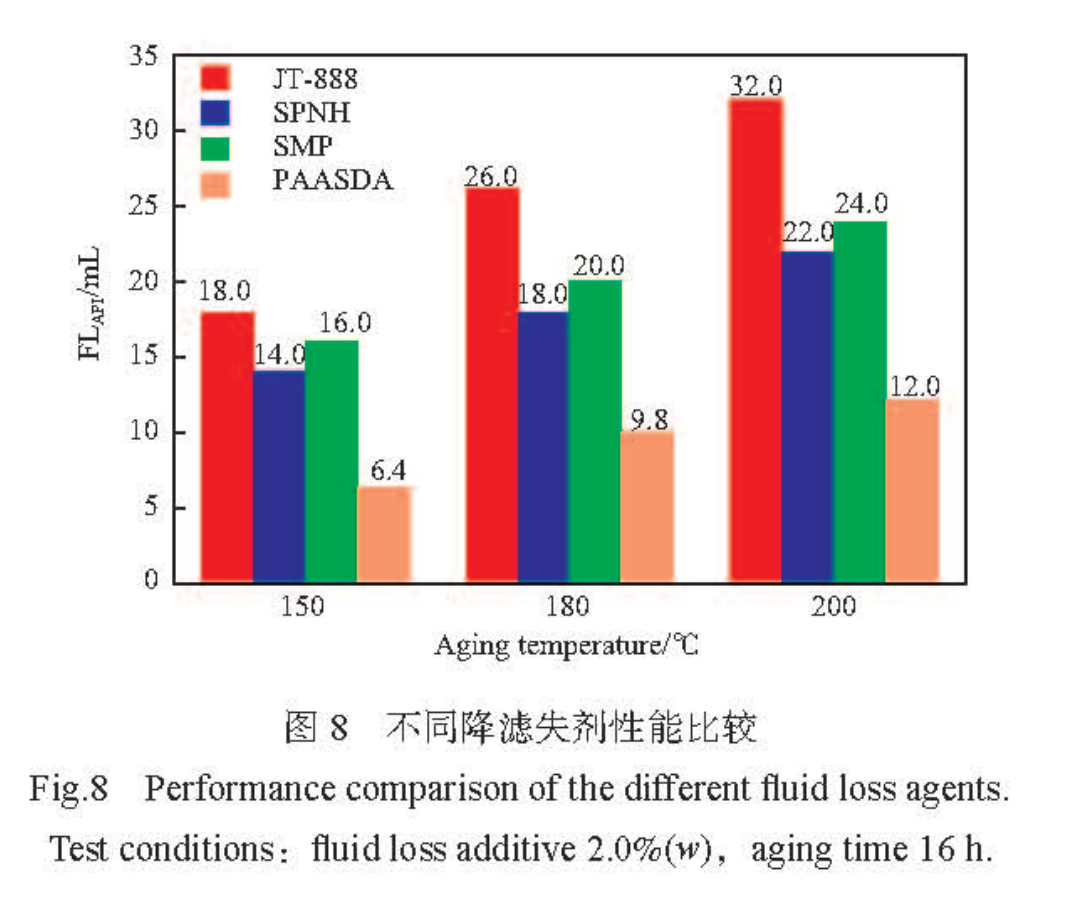
At a dosage of 2.0% (w) of water loss agent, a comparison of aging experiments was conducted in freshwater slurry at different temperatures using zwitterionic polymer water loss agent JT-888, sulfonated lignite resin water loss agent SPNH, sulfonated phenolic resin water loss agent SMP, and synthesized water loss agent PAASDA. The results are shown in Figure 8. From Figure 8, it can be seen that at different aging temperatures, the filtration loss of PAASDA is 6.4, 9.8, and 12mL, which is significantly lower than other sulfonated fluid loss agents, indicating that the synthesized zwitterionic polymer PAASDA is an excellent fluid loss agent with excellent filtration performance.
2.2.5 Composite Saltwater Polysulfonate Drilling Fluid
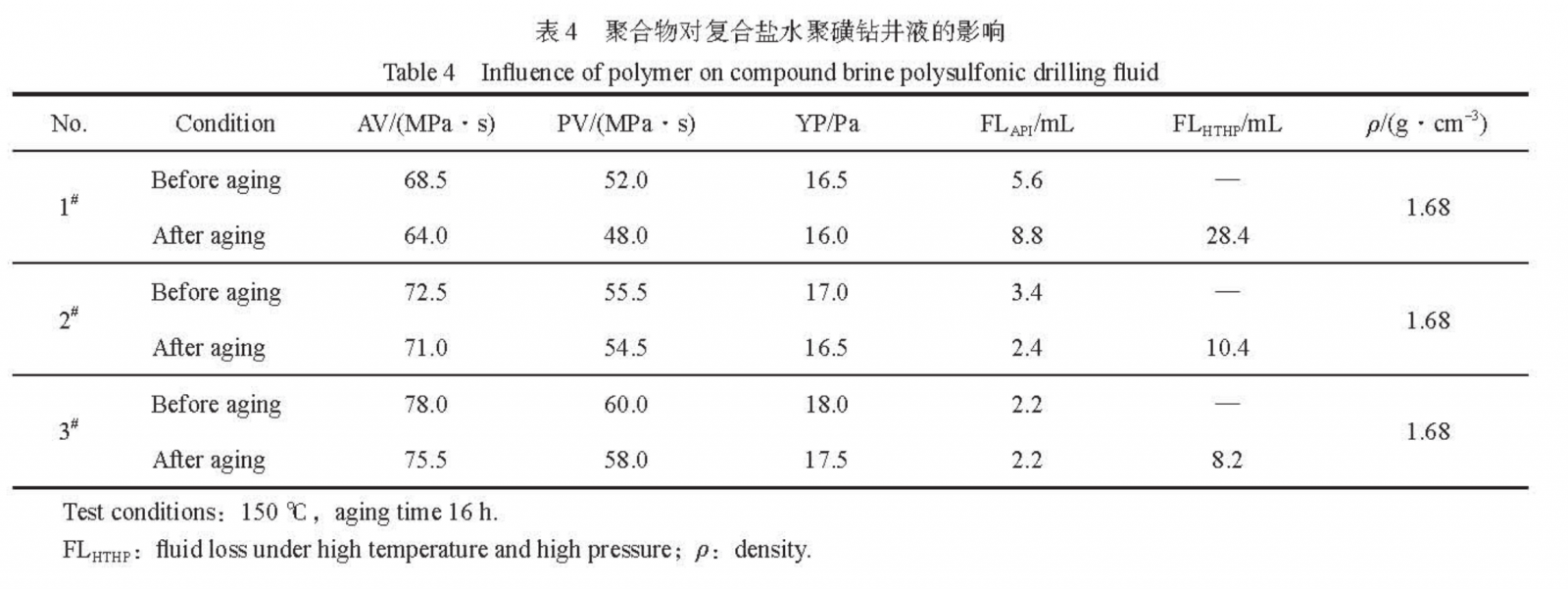
After hot rolling the composite salt water polysulfonate drilling fluid at 150℃ for 16 hours, the FLAPI and rheological parameters were measured, and the FLHTHP was measured at 150℃ and 3.5 MPa. The results are shown in Table 4. From Table 4, it can be seen that PAASDA exhibits superior performance in the composite saltwater polysulfonate drilling fluid system compared to the conventional drilling fluid loss agent HL-60. Replacing HL-60 with PAASDA, after aging at the same dosage, the FLAPI of the composite saline polysulfonate drilling fluid decreased from 8.8mL to 2.4mL, with a decrease of 72.7%; FLHTHP decreased from 28.4mL to 10.4mL, with a decrease of 63.4%. After increasing the dosage of PAASDA and aging, FLAPI decreased to 2.2mL and FLHTHP decreased to 8.2mL, with stable rheological properties and no thickening phenomenon. This indicates that PAASDA can be more effectively applied in composite saline polysulfonate drilling fluid systems, and the filtration loss reduction effect can also be improved with increasing dosage.
2.2.6 Microscopic Morphology Analysis of Filter Cake
Selecting fresh water slurry without PAASDA and with a PAASDA dosage of 2.0% (w) for SEM characterization, the results are shown in Figure 9. From Figure 9, it can be seen that a large number of clay particles aggregate on the surface of the filter cake of freshwater slurry, and there are obvious pores and cracks on the surface of the filter cake, which become the main channel for water loss during the filtration process. After adding PAASDA, a dense filter cake was formed in the freshwater slurry, without obvious water loss pores. The small amount of granular protrusions and wrinkles on the surface were also well connected to the lower substrate. This indicates that the mud cake structure formed by clay particles in the polymer based slurry system is dense, and the clay particles are well dispersed in the system, effectively reducing the filtration loss of the system.

3.Conclusions
1) The suitable reaction conditions for synthesizing PAASDA using free radical aqueous solution polymerization method with ammonium persulfate sodium bisulfite as initiator system are: m (AM): m (AMPS): m (SSS): m (AA): m (DMDAAC)=30:40:8:15:7, and initiator dosage of 0.6% (w). The optimal dosage of PAASDA obtained by using this ratio in saline based slurry is 2.0% (w). After aging at 150℃, the FLAPI is 8.0mL.
2) Compared with commonly used sulfonation fluid loss agents JT-888, SPNH, and SMP in oil fields, PAASDA has better filtration performance. In the composite salt water polysulfonate drilling fluid system, PAASDA replaced HL-60 for better water loss reduction effect, and there was no thickening phenomenon when the filtration loss was increased.
3) Adding PAASDA makes the drilling fluid filter cake denser, with more complete molecular chains and better filtration loss reduction performance.



