Case Studies
Case Studies
- Construction of A New Organic-inorganic Composite Emulsion and Its Enhanced Mechanical Properties of Oil Well Cement(Part 1)
- Construction of A New Organic-inorganic Composite Emulsion and Its Enhanced Mechanical Properties of Oil Well Cement(Part 2)
- The Application Prospects of DeepSeek Large Model in Petroleum Engineering(Part 1)
- The Application Prospects of DeepSeek Large Model in Petroleum Engineering(Part 2)
- Development and Performance Evaluation of Efficient Asphalt Dispersant (Part 1)
- Development and Performance Evaluation of Efficient Asphalt Dispersant (Part 2)
- Development and Performance Evaluation of Efficient Asphalt Dispersant (Part 3)
- Research and Application Status of Drilling Fluid Plugging Materials (Part 1)
- Research and Application Status of Drilling Fluid Plugging Materials (Part 2)
- Research and Application Status of Drilling Fluid Plugging Materials (Part 3)
2.4 Apparent Viscosity Analysis
In order to study the influence trend of the nanocomposite material solution ADC16/OPRT and pure copolymer solution ADC16 on the apparent viscosity, this article studied the differences in its apparent viscosity from the perspectives of temperature resistance and shear resistance.
(1) .Temperature Resistance
The apparent viscosity of ADC16 solution and ADC16/OPRT solution were measured within the temperature range of 30-150℃, and the results are shown in Figure 5.
From the overall trend of the temperature effect on the sample solution in Figure 5, it can be seen that as the temperature increases, the apparent viscosity decreases. This is because polymers mainly form reversible supramolecular structures through strong Van Der Waals forces, and a large number of associated groups aggregate, causing polymer segments in the aqueous solution to intertwine through hydrogen bonding. However, as the temperature increases, the stability of intermolecular interactions decreases, making it prone to degradation to some extent. At any temperature, the apparent viscosity of ADC16/OPRT solution is always higher than that of ADC16 solution, and the viscosity retention rate is 43.2% at 30-150℃, indicating that its aqueous solution still has good temperature resistance characteristics.
.jpg)
(2) .Shear Resistance
The variation curves of the apparent viscosity and shear rate of ADC16 solution and ADC16/OPRT solution are shown in Figure 6.
From Figure 6, it can be seen that as the shear rate increases from 1s-1 to 500s-1, the apparent viscosity of the sample solution gradually decreases, and the overall behavior shows shear thinning. This phenomenon is caused by the instantaneous "depolymerization" of polymer macromolecules or the destruction of group cross-linking structure under shear action. However, at the same shear rate, the shear degradation of OPRT/ADC16 in aqueous solution is lower than that of pure copolymer ADC16. This is because the introduction of bentonite nanolayer structure enhances intermolecular hydrophobic association, making the three-dimensional network structure of the polymer more stable and less susceptible to shear damage. Therefore, ADC16/OPRT nanocomposites have excellent shear resistance.
.jpg)
2.5 Analysis of Viscoelastic Behavior
Viscoelastic polymer aqueous solutions belong to pseudoplastic non Newtonian fluids and can exhibit both viscous and elastic rheological properties. This article investigates the viscoelastic behavior of pure polymer and nanocomposite aqueous solutions, and the measurement results are shown in Figure 7.
From Figure 7, it can be seen that as the oscillation frequency of the solution increases, both G' and G'' of the sample show a gradual increasing trend. The G' and G'' of composite material ADC16/OPRT are always greater than those of pure copolymer ADC16, showing better viscoelasticity. In addition, the G' and G'' of composite material ADC16/OPRT have a critical frequency value at lower frequency values, and the performance of aqueous solutions is similar to that of ordinary viscous fluids, with lower viscosity; When it exceeds the critical frequency value, G' is greater than G'',the three-dimensional network structure is stronger. This phenomenon is caused by the dominant role of elasticity in the solution. These results can be explained as follows: due to the unique molecular structure in composite materials, the electrostatic and hydrogen bonding interactions between the matrix molecular segments and the stripping layer in bentonite strengthen the hydrophobic association between the original molecules, forming a more complex and stable three-dimensional spatial grid structure.
.jpg)
2.6 Hydrophobic Association Characteristics of Composite Materials
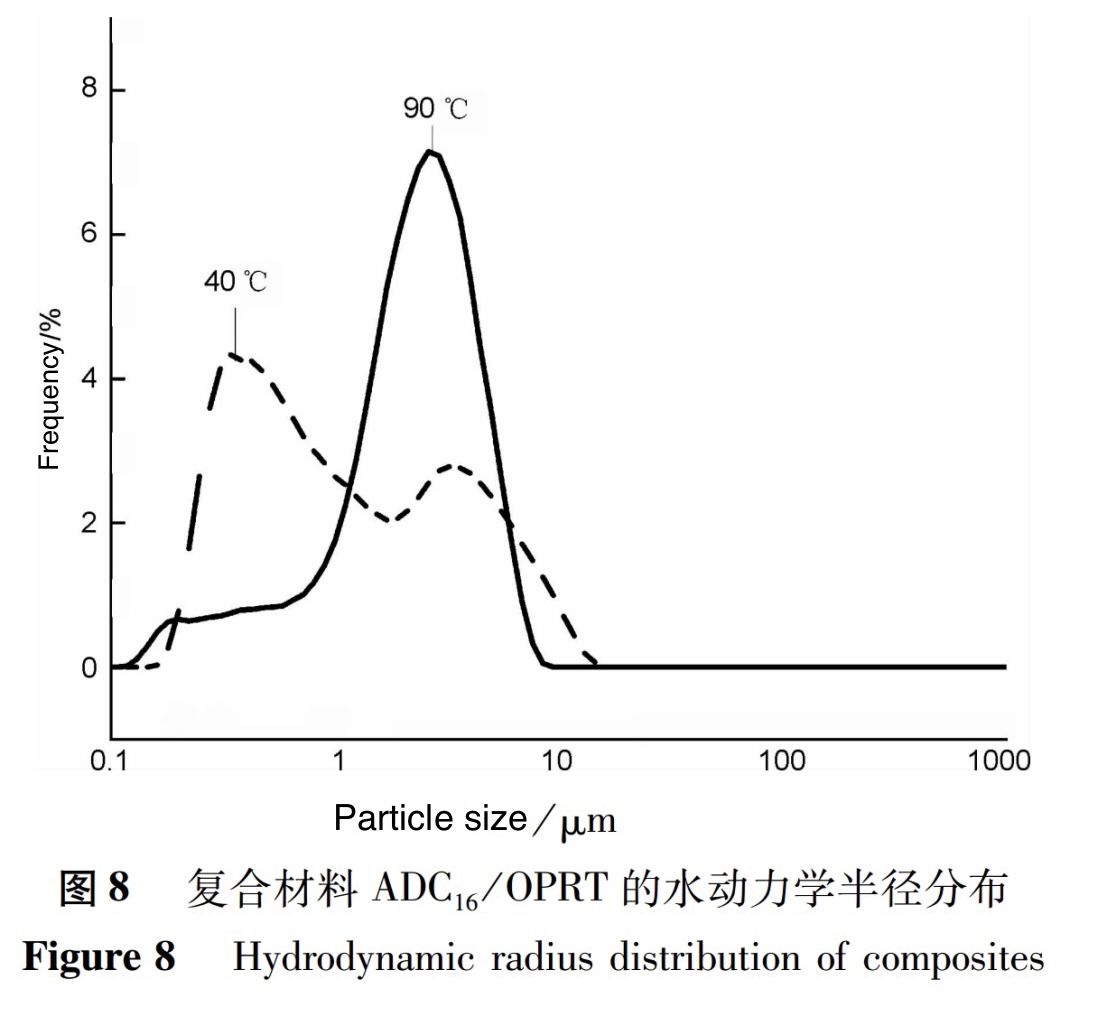
The viscosity of solutions with a "hydrophobic effect" is mainly composed of unstructured viscosity, which is determined by the hydrodynamic size of polymer molecules. The hydrodynamic size of polymers is not only a factor in the generation of flow resistance, but also a necessary condition for the formation of structures. To further understand the structural transformation of hydrophobic association in the composite solution system under temperature changes, the hydrodynamic radius of the solution system was measured using the DLS method, and the test results are shown in Figure 8.
From the measurement results, it can be seen that there are different hydrodynamic radius peaks in the two temperature systems, corresponding to different types of aggregate structures in the composite material solution. At an ambient solution temperature of 40℃, the peak at Rh=0.33um exhibits an intramolecular association structure of the polymer; At Rh=3.46um, there is a relatively small amount of intermolecular association structure; As the temperature of the environmental solution increases, when the temperature is 90℃, the peak value at Rh=3.46um in the hydrodynamic radius distribution of the composite material shifts to the left, and a significantly increased peak appears at Rh=2.72um, indicating a more concentrated distribution of polymer fluid volume. This phenomenon indicates that as the temperature increases and the solvation of the solution further strengthens, the solution is fully dissolved, thereby promoting the generation of intermolecular entanglement and cooperation between molecular chains. With the increase of temperature, the reconstruction of intermolecular association structure forms a more uniform aggregate structure. The links between aggregates can form a uniform, three-dimensional network structure that covers the entire system in the polymer, thus composite materials have unique rheological properties.
3. Conclusion
This article uses in-situ polymerization method to prepare water-soluble hydrophobic association polymer-bentonite nanocomposites (ADC16/OPRT). Through infrared spectroscopy and XRD analysis, it was confirmed that OPRT was successfully dispersed in the ADC16 copolymer matrix in the form of exfoliation and intercalation structures. The TGA results indicate that the thermal stability of ADC16/OPRT is superior to that of pure ADC16. The study of solution performance shows that under the same conditions, the temperature resistance, shear resistance, and viscoelasticity of ADC16/OPRT solution have been improved compared to pure ADC16. In addition, the composite material has certain hydrophobic association properties. Therefore, ADC16/OPRT nanocomposites have good application prospects in the petrochemical industry.



