Case Studies
Case Studies
- Construction of A New Organic-inorganic Composite Emulsion and Its Enhanced Mechanical Properties of Oil Well Cement(Part 1)
- Construction of A New Organic-inorganic Composite Emulsion and Its Enhanced Mechanical Properties of Oil Well Cement(Part 2)
- The Application Prospects of DeepSeek Large Model in Petroleum Engineering(Part 1)
- The Application Prospects of DeepSeek Large Model in Petroleum Engineering(Part 2)
- Development and Performance Evaluation of Efficient Asphalt Dispersant (Part 1)
- Development and Performance Evaluation of Efficient Asphalt Dispersant (Part 2)
- Development and Performance Evaluation of Efficient Asphalt Dispersant (Part 3)
- Research and Application Status of Drilling Fluid Plugging Materials (Part 1)
- Research and Application Status of Drilling Fluid Plugging Materials (Part 2)
- Research and Application Status of Drilling Fluid Plugging Materials (Part 3)

Abstract
A new type of water-soluble hydrophobic association polymer - bentonite nanocomposite was prepared by in-situ polymerization using organic modified bentonite, 2-acrylamide 2-methylpropane sulfonic acid (AMPS), N, N-dimethylacrylamide (DMAA), and quaternary ammonium salt monomer D-C16Br as raw materials. The product structure was characterized by infrared spectroscopy and X-ray diffraction, and the effect of the introduction of nanoclay materials on the performance of the copolymer was studied compared with pure copolymer AMPS/DMAA/D-C16Br. The results show that under the promotion of hydrophobic association structure of polymer molecules by bentonite, composite materials with composite intercalation structures have better thermal stability, temperature resistance, shear resistance, and viscoelasticity than pure polymers, indicating that this nanocomposite material has good application prospects compared to pure copolymers.
In recent years, the synthesis of nanocomposites composed of inorganic clay and organic polymer matrices at the nanoscale has become a hot topic in the fields of materials science and petrochemical industry. Incorporating nano clay fillers into organic polymer matrices can significantly improve the strength, modulus, and thermal stability of nanocomposites, as well as reduce permeability. Inorganic bentonite is a type of layered silicate clay mineral mainly composed of montmorillonite. Its structure is characterized by easy intercalation and peeling, large specific surface area, strong cation exchange ability, and large aspect ratio, making it easier to form interlayer complexes with various organic molecules. Due to the hydrophilic properties of bentonite, in order to improve its compatibility with organic polymer matrices, surfactants containing organic ammonium ions are usually used to modify it through ion exchange reactions with Na+/Ca2+, allowing the crystal structure of the clay layer to be embedded or peeled off in the polymer matrix to form polymer nanocomposites. In addition, the interaction between organic and inorganic phases can endow nanocomposites with excellent temperature resistance and mechanical properties.
At present, a large amount of research has been conducted on polymer/clay nanocomposites in various fields. For example, Hu et al. obtained copolymers with better temperature and salt resistance using polyacrylamide/MMT nanocomposites; Zhu et al. investigated the synthesis and thermal properties of polyaniline clay nanocomposites (PACN), and the results showed that the addition of modified clay nanoparticles improved the temperature resistance of the pure copolymer and significantly increased the melting point of the composite material. Based on the above research, this article proposes a preparation method for water-soluble hydrophobic association polymer/modified nanocomposites based on the flaky structure and high thermal stability of montmorillonite in bentonite. Sodium based bentonite was organically modified as an active polymer filler/comonomer, and a composite material (Scheme 1) was prepared by in-situ intercalation method. The thermal stability, water solution temperature resistance, shear resistance, viscoelastic behavior, and hydrophobic association characteristics of the composite material molecules were comprehensively studied.
.png)
1.Experimental Part
1.1Instruments and Reagents
WQF520 infrared spectrometer; X'Pert MPD PRO type X-ray diffraction energy spectrometer; DSC 823 TGA/SDTA85/e thermogravimetric analyzer; HAAKE MARS high-temperature rheometer; BI-200SM laser scattering system.
2-acrylamide-2-methylpropanesulfonic acid (AMPS), N-N dimethylacrylamide (DMAA), dimethylaminoethyl methacrylate (DMAEMA), bromocectane, acetone, ether, hexadecyltrimethylammonium chloride (CTAB), azodiisobutyronitrile (AIBN); Sodium based bentonite (200 mesh ion exchange capacity (CEC): 0.5mmol/100g).
1.2 Synthesis
(1) Synthesis of Quaternary Ammonium Monomer (D-C16Br)
Mix accurately weighed 1.000g of dimethylaminoethyl methacrylate (DMAEMA) with a small amount of acetone solvent and add to a three necked flask. Add 0.002g of hydroquinone as an inhibitor and preheat the three necked flask in an oil bath that has been heated to 50℃. Add 1.000g of bromocectane to a constant pressure funnel, then slowly drip it into a mixed solution of dimethylaminoethyl methacrylate and acetone, and perform condensation reflux at a temperature of 50℃. After the addition of brominated hexadecane, continue the reaction for 10 hours to obtain a viscous solution. The solution obtained from the reaction was backwashed in a non-polar solvent ether to remove monomers and polymerization inhibitors. After vacuum drying, a white crystalline product D-C16Br was obtained.
(2) Preparation of Organic Intercalation Modified Bentonite (OPRT)
Add 5.000g of sodium based bentonite Na PRT and 100mL of deionized water to a three necked flask, stir in an 80℃ oil bath for 30 minutes to evenly disperse and form a bentonite suspension. Mix 1.200g of intercalating agent CTAB with 20mL of deionized water to form a milky white solution, slowly add it to the bentonite dispersion system, and continue stirring for 8 hours. After the intercalation reaction is completed, it is allowed to stand for 12 hours and centrifuged at 4500rpm for 5 minutes. The centrifuged organic bentonite is washed with deionized water until there is no Br-, and then dried and crushed in a vacuum oven to obtain a relatively pure organic bentonite sample.
(3) Synthesis of Organic Intercalation Modified Bentonite Nanocomposites (ADC16/OPRT)
In situ polymerization is used to synthesize organic intercalated modified bentonite nanocomposites. In a 250mL beaker, first dissolve the monomers to form a uniform solution according to AMPS: DMAA: D-C16Br=10.0:8.0:0.1 (mass ratio), and adjust the pH to about 7. Add the prepared organic bentonite (1.0% of the total mass of monomers) to the solution under high-speed magnetic stirring, and continue stirring at room temperature for 2 hours to ensure that the monomers are fully adsorbed on the bentonite nano layer. Add initiator AIBN (0.5% of the total mass of monomer and organic bentonite) to the solution system and react for 5 hours at 70℃. The entire reaction process is carried out under nitrogen protection. Place the reaction products in a static state and layer them, take the lower layer products for filtration, washing, and drying (Figure 1).
.jpg)
2. Results and Discussion
2.1 Infrared Structural Characterization
Infrared spectroscopy was performed on pure sodium based bentonite and its nanocomposites, and the results are shown in Figure 2. From the test results, it can be seen that the absorption band of sodium based bentonite in Figure 2 (a) at 3632cm-1 is the stretching vibration peak of -OH in the bentonite; 3440cm-1 is the stretching vibration absorption peak of interlayer water; The characteristic peak at 1635cm-1 is attributed to the H-O-H bending vibration in water molecules, which also exists in the interlayer structure of the bentonite layer; The absorption peaks at 1041cm-1 and 793cm-1 are Si-O tensile vibration and Mg-Al-OH bending vibration absorption bands, respectively.
Figure 2 (b) shows the infrared spectrum of modified bentonite nanocomposite material ADC16/OPRT. The typical strong absorption peaks at 2920cm-1, 2835cm-1 and 1468cm-1 are the stretching vibration peaks of -CH2 and -CH3, belonging to the copolymer main chain and long chain alkyl structure, proving that the quaternary ammonium salt cationic macromonomer D-C16Br has entered the interlayer structure; The absorption peak at 1645cm-1 confirmed the existence of C=O bond in DMAA; The strong absorption peak at 1042cm-1 and the characteristic peak at 632cm-1 are the stretching vibration peaks of the sulfonic group S-O and C-S in AMPS, respectively. In the infrared spectrum of Figure 2 (b), the absorption bands of Si-O tensile vibration and Mg-Al-OH bending vibration still exist, indicating that the layered silicate structure did not undergo transformation after the displacement of cations and organic substances between the bentonite layers.
Based on the above spectral analysis and comparison, it can be concluded that the target nanocomposite material (ADC16/OPRT) has successfully polymerized.

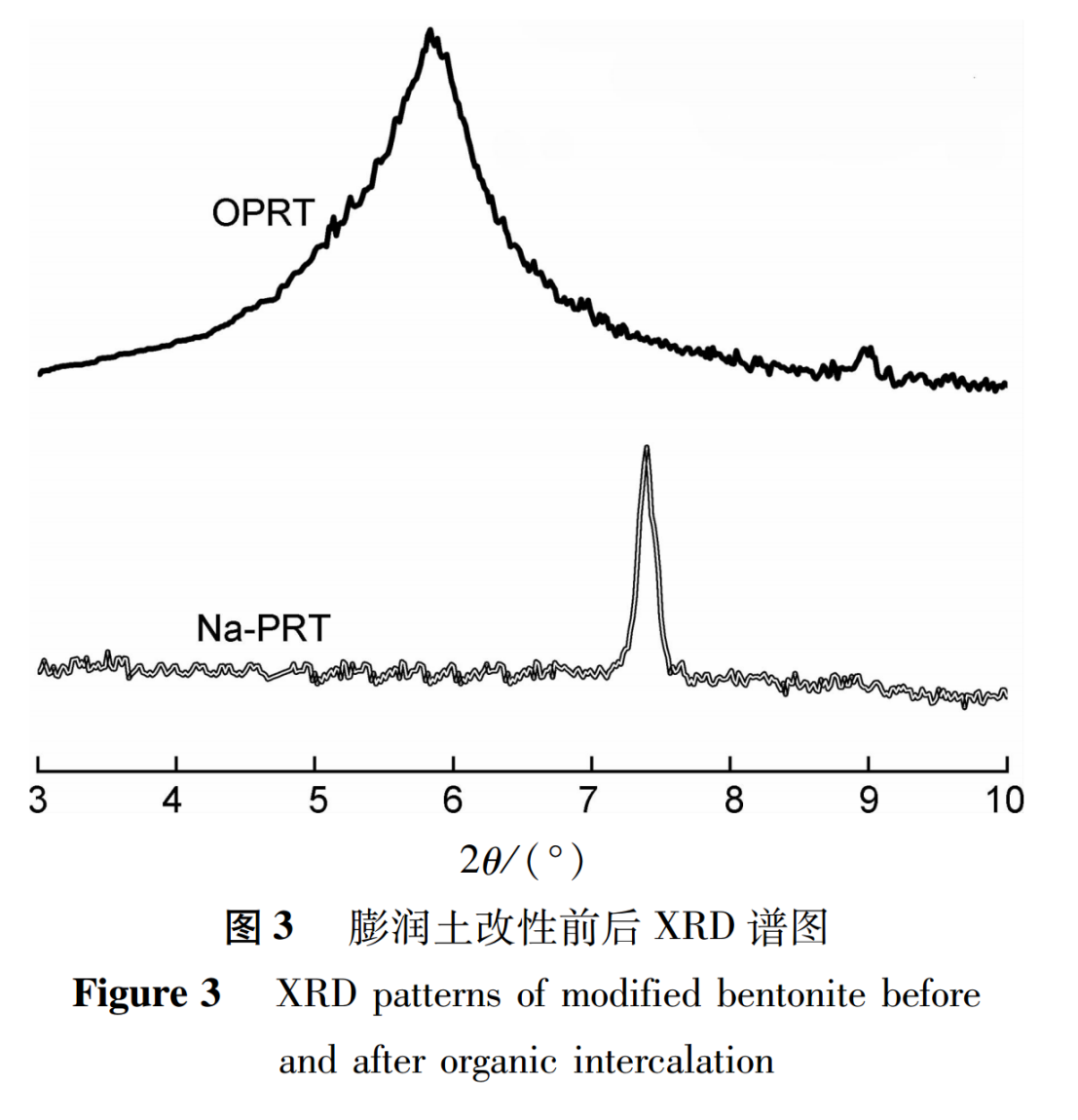
2.2 XRD Characterization
The X-ray crystal plane (001) diffraction peak in the XRD spectrum is the basis for determining the existence of interlayer structure, and is also an important method for determining whether organic polymer chains are inserted into the interlayer structure of the bentonite layer. When the interlayer spacing in the layered structure of bentonite increases due to the insertion of molecules, the characteristic peaks of X-ray diffraction will shift towards low angles, the peak intensity will decrease, and the peak deformation will be wide.
The XRD test spectra of unmodified sodium bentonite (Na PRT) and organic modified bentonite (OPRT) are shown in Figure 3. From Figure 3, it can be seen that the diffraction peak of Na-PRT is at 7.3º(2θ),the diffraction peak of OPRT shifts forward to 5.8º(2θ). According to the Bragg equation calculation, the interlayer spacing of Na-PRT and OPRT is 1.21 and 1.52, respectively.
The above analysis of results indicates that the interlayer spacing of OPRT significantly increases, indicating that the organic modification preparation has been successful.
2.3 Thermal Stability Analysis
The effect of OPRT on the thermal stability of copolymers was studied using TGA. The TGA curves of pure copolymer ADC16 and ADC16/OPRT nanocomposites are shown in Figure 4.
As shown in Figure 4, the weight loss curves of pure copolymer ADC16 and nanocomposite material ADC16/OPRT exhibit a three-step degradation trend. In the first thermal degradation stage between 95.2 and 106.8℃, the degradation temperature and mass loss of the two samples are approximately the same, about 5.3%; In the second thermal degradation stage, where the mass loss is the fastest, the decomposition temperatures of the pure copolymer sample and the composite material ADC16/OPRT are 284℃ and 364℃, respectively. The weight loss during this stage is about 39.7% and 36.5%, respectively; In the third thermal degradation stage, the thermal degradation temperature of ADC16/OPRT increased from 452℃ to 534℃ compared to pure copolymers. This stage is mainly due to the breakage of the polymer main chain and the volatilization of small molecules.
The above phenomenon indicates that the addition of organic modified bentonite significantly improves the thermal stability of the polymer and reduces its mass loss during the same thermal degradation stage. This is mainly due to the rigidity and thermal stability characteristics of inorganic materials inherent in bentonite. In addition, the layered structure presented by its dispersion in the polymer matrix can ,to some extent, hinder the contact between oxygen and the polymer, slowing down basic heat transfer at high temperatures, thereby reducing the decomposition rate of the polymer and delaying the volatilization of the decomposition products.
.jpg)



