Case Studies
Case Studies
- Construction of A New Organic-inorganic Composite Emulsion and Its Enhanced Mechanical Properties of Oil Well Cement(Part 1)
- Construction of A New Organic-inorganic Composite Emulsion and Its Enhanced Mechanical Properties of Oil Well Cement(Part 2)
- The Application Prospects of DeepSeek Large Model in Petroleum Engineering(Part 1)
- The Application Prospects of DeepSeek Large Model in Petroleum Engineering(Part 2)
- Development and Performance Evaluation of Efficient Asphalt Dispersant (Part 1)
- Development and Performance Evaluation of Efficient Asphalt Dispersant (Part 2)
- Development and Performance Evaluation of Efficient Asphalt Dispersant (Part 3)
- Research and Application Status of Drilling Fluid Plugging Materials (Part 1)
- Research and Application Status of Drilling Fluid Plugging Materials (Part 2)
- Research and Application Status of Drilling Fluid Plugging Materials (Part 3)

The use of supercritical water (SCW) for heavy oil upgrading can degrade large molecules into small molecules, reducing viscosity and removing heteroatom, demonstrating advantages that traditional thermal cracking does not have in many aspects. This article introduces the molecular structure and thermal cracking mechanism of heavy oil. Polycyclic aromatic hydrocarbons (PAHs) are the main components of heavy oil, and the generation and transformation of PAHs during pyrolysis have a decisive impact on the results of heavy oil upgrading. This article reviews the mechanism of SCW modification of heavy oil, analyzes the influence of SCW on PAHs changes from three aspects: water molecule dissolution, cluster "cage effect", and hydrogen supply capacity, and discusses other potential supercritical fluids that can be used for heavy oil modification. Based on this, future research directions are discussed.
With the consumption of light petroleum resources, the utilization of heavy oil has become increasingly important. The abundant natural oil sands, abundant sources of vacuum residue, and coal to oil are the main sources of heavy oil. Effectively utilizing heavy oil resources is of great significance for maintaining energy supply. Heavy oil has a higher content of gum and asphaltene, so its quality is inferior to conventional petroleum. The high viscosity, high molecular weight, and high content of heteroatoms limit the effective utilization of heavy oil. Heavy oil upgrading (also known as heavy oil lightweighting) is the process of cracking large molecules in heavy oil into small molecules. The traditional heavy oil upgrading methods are divided into thermal cracking and hydrocracking, which respectively face the problems of severe coking and catalyst deactivation. Compared to thermal cracking, using supercritical water (SCW) for heavy oil upgrading results in higher oil conversion rate, less coking, and better oil quality. The process of SCW upgrading heavy oil does not require the addition of additional catalysts and solvents, nor does it generate excess waste, resulting in minimal environmental impact and in line with the principles of sustainable development.
SCW refers to the high-temperature and high-pressure state where water is above the critical point (22.1 MPa, 374℃). At this time, water has many unique physical properties, with a density closer to that of a liquid but a diffusion coefficient closer to that of a gas. Polycyclic aromatic hydrocarbons (PAHs) are widely present in coal and petroleum, and are organic compounds composed of several benzene rings, which may have branched chain structures. Light components come from the breakage of branched chains, while heavy components come from the condensation of multiple aromatic rings. Therefore, the generation and transformation of PAHs during the cracking process have a significant impact on the distribution of cracking products.
At present, although there are some studies on SCW modified heavy oil, there is still a lack of literature on the mechanism of SCW modified heavy oil from the perspective of the influence of water molecules on the generation and conversion of PAHs. This article introduces the molecular structure and thermal cracking mechanism of heavy oil, summarizes the mechanism of SCW modified heavy oil, focuses on the changes in PAHs during the SCW modified heavy oil process, and conducts a systematic analysis from three aspects: water molecule dissolution, water molecule clustering, and hydrogen supply capacity. At the same time, other supercritical fluids with potential applications for heavy oil modification are proposed, and based on this, future research directions are discussed.
1.Molecular Structure and Thermal Cracking Mechanism of Heavy Oil
Heavy oil can be divided into four components: saturated fraction, aromatic fraction, resin, and asphaltene. Compared to light crude oil, heavy oil contains more gum and asphaltene. Colloids and asphaltenes are composed of multiple aromatic rings and branched chains surrounding multiple aromatic rings, with the main components being PAHs and similar molecular structures. The difference is that asphaltene has a larger molecular weight and more aromatic rings. If a molecule contains only one polyaromatic ring, it is called an "island" like molecule; if a molecule contains multiple polyaromatic rings connected by chain hydrocarbons, it is called an "archipelago" like molecule.
Due to the presence of multiple aromatic rings, molecules of resin and asphaltene will aggregate through π-π stacking forces. In addition, hydrogen bonding, acid-base interactions, metal coordination, hydrophobic interactions, and other interactions between resin and asphaltene molecules can also lead to molecular aggregation. In a stable environment, the steric hindrance between the branches restricts the unrestricted aggregation of molecules, allowing gum and asphaltene molecules to stably distribute in heavy oil. The complex molecular structure of heavy oil leads to complex intermolecular interactions.
The thermal cracking process of heavy oil follows a free radical reaction mechanism. The structure of resin and asphaltene can infer the mechanism of light components and coking formation during the thermal cracking process of PAHs, as shown in Figure 1. From Figure 1, it can be seen that during the thermal cracking process, PAHs molecular chains break to form chain hydrocarbon radicals and new PAHs radicals. Among them, chain hydrocarbon radicals (including those formed by the reaction of saturated hydrocarbons and aromatics in heavy oil) can receive hydrogen atoms to form chain hydrocarbons, thereby lightening heavy oil and forming light component products (Route 1). But as the thermal cracking deepens, the free radicals of chain hydrocarbons can crack to form smaller alkanes and unsaturated hydrocarbons. As the degree of unsaturation deepens, unsaturated hydrocarbons may dehydrogenate and condense into rings, forming cyclic alkanes, aromatics, and PAHs, which are the products of recombination. Li et al. found a competitive relationship between cracking and dehydrogenation condensation processes. When the reaction conditions are severe, dehydrogenation condensation is the dominant reaction, and vice versa, cracking is the dominant reaction. It is worth noting that there is also ring opening cleavage of PAHs in the reaction system, which achieves the conversion of heavy components to light components.
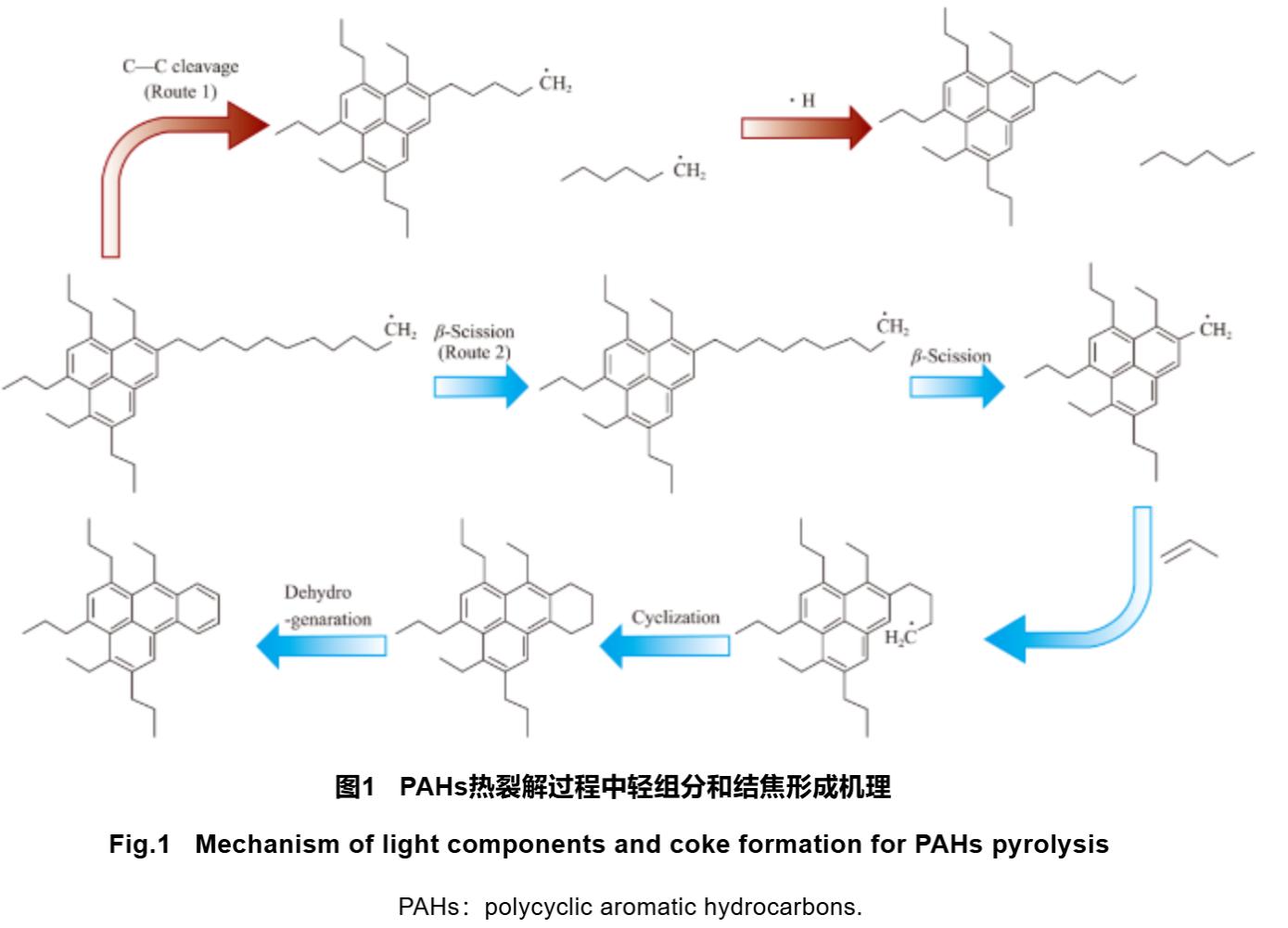
At present, the research on the mechanism of PAHs generation during the cracking process is relatively detailed. The Frenklach model and Bittner Howard model suggest that the first benzene ring is formed by the reaction of alkyne radicals, which then continue to react and cyclize with alkyne radicals, forming PAHs by increasing the number of aromatic rings. The Melius model suggests that two 5-membered ring radicals condense to form two 6-membered rings, which further cyclize to form PAHs. Van Speybroeck et al. estimated through transition state theory and density functional theory (DFT) that the reason for the formation of PAHs is the addition of ethylbenzene radicals and ethylene to form butylbenzene radicals and cyclization. Liu et al. inferred from thermodynamic parameters that methylated aromatic radicals and short olefin radicals are highly active components for generating coke. The above mechanisms all suggest that the addition and cyclization of small molecule hydrocarbon radicals (including alkynes, olefins, and monocyclic hydrocarbons) with benzene or multiple benzene rings is the reason for the increase in the number of aromatic rings in PAHs and ultimately leading to coking. The coking pathway inferred by Liu et al. is shown in Figure 1, Route 2, where the generation and transformation of PAHs include: formation of methylated aromatic radicals, addition of methylated aromatic radicals to olefins, cyclization of aromatic radicals, and dehydrogenation of cyclized aromatic radicals. As cyclization and dehydrogenation continue, the number of aromatic rings in PAHs increases, ultimately leading to coking.
2. Mechanism of SCW Modified Heavy Oil
Compared with the thermal cracking system of heavy oil, the SCW modified heavy oil system contains water molecules. The interaction between water molecules and crude oil molecules causes changes in the cracking of crude oil molecules, and some reactions are strengthened, such as the cracking of large molecular chain hydrocarbons into small molecular chain hydrocarbons, while some reactions are weakened, such as the condensation and coking of PAHs molecules. Under the combined effect of these influences, the generation reaction of PAHs molecules is inhibited during the cracking process, while the conversion reaction of PAHs is strengthened, ultimately leading to a decrease in the content of heavy components and an increase in the content of light components. The current research mainly explores the influence of water molecules through experiments and molecular dynamics simulations. The conclusions obtained from the experiment are more accurate, but there are certain limitations in using them to elucidate the mechanism. Molecular dynamics simulation has the advantage of not being affected by external factors and can directly reveal the internal changes of molecules. It can serve as an important supplement to traditional experiments in the study of heavy oil upgrading. However, it is worth noting that due to limitations in computer computing power, the number of simulated molecules is much smaller than the actual number of molecules. Therefore, it is necessary to combine simulation results with experimental results to explore the molecular changes during the SCW modified heavy oil process.
Based on experimental and simulation results, the influence of water molecules on the cracking process of heavy oil molecules was sorted out from three aspects, namely the dissolution effect of water molecules on heavy oil molecules, the formation of clusters of water molecules dispersed around heavy oil molecules to produce a "cage effect", and water molecules acting as hydrogen donors.
2.1 Dissolution of Water Molecules
SCW is a special phase of water, where high pressure exceeding the critical point of water prevents SCW molecules from being in the gaseous state, while high temperature exceeding the critical point of water results in SCW molecules having greater energy than liquid water. Therefore, the interaction between SCW molecules has the characteristic of being between gas and liquid. The presence of intermolecular hydrogen bonds in liquid water is an important reason why oil is insoluble in water. When water reaches the supercritical state, the hydrogen bond content rapidly decreases, and the solubility of oil in SCW increases significantly. However, asphaltene and resin cannot be completely dissolved in SCW, thus forming oil and water phases.
The main reactions that occur in the oil and water phases during the SCW upgrading process of heavy oil are shown in Figure 2. As shown in Figure 2a, coking reaction mainly occurs in the oil phase. This is because during the reaction process, large molecules of PAHs are mainly distributed in the oil phase, and the concentration of PAHs free radicals is high, resulting in a high coking reaction rate. Small molecule hydrocarbon radicals are mainly distributed in the aqueous phase, avoiding the dehydrogenation condensation of small molecule hydrocarbon radicals to generate PAHs. When large molecule PAHs are cracked, the distribution coefficients of unsaturated hydrocarbons and some smaller molecular weight PAHs in the aqueous phase are greater than their distribution coefficients in the oil phase. This leads to the rapid extraction of small molecule PAHs into the aqueous phase, and the coking reaction requires the participation of small molecule hydrocarbons. Therefore, the coking reaction in the oil phase is inhibited. At the same time, some small molecules of PAHs enter the water phase from the oil phase, causing a decrease in the concentration of PAHs in the oil phase and a decrease in the possibility of direct condensation and coking between PAHs. After entering the aqueous phase, the concentration of hydrocarbon radicals decreases significantly under the dilution of water molecules. Water molecules can also provide hydrogen atoms for hydrocarbon radicals to saturate them, thereby terminating the reaction. In summary, the redistribution of hydrocarbon free radicals caused by SCW makes PAHs containing fatty branched chains more prone to chain breakage to form fatty hydrocarbons and aromatics, while the generated small molecule hydrocarbons are transferred, inhibiting the reaction of small molecule generation of PAHs and the reaction of PAHs aromatic ring increase. Therefore, during the process of SCW upgrading heavy oil, the yield of light components increases and coking decreases.
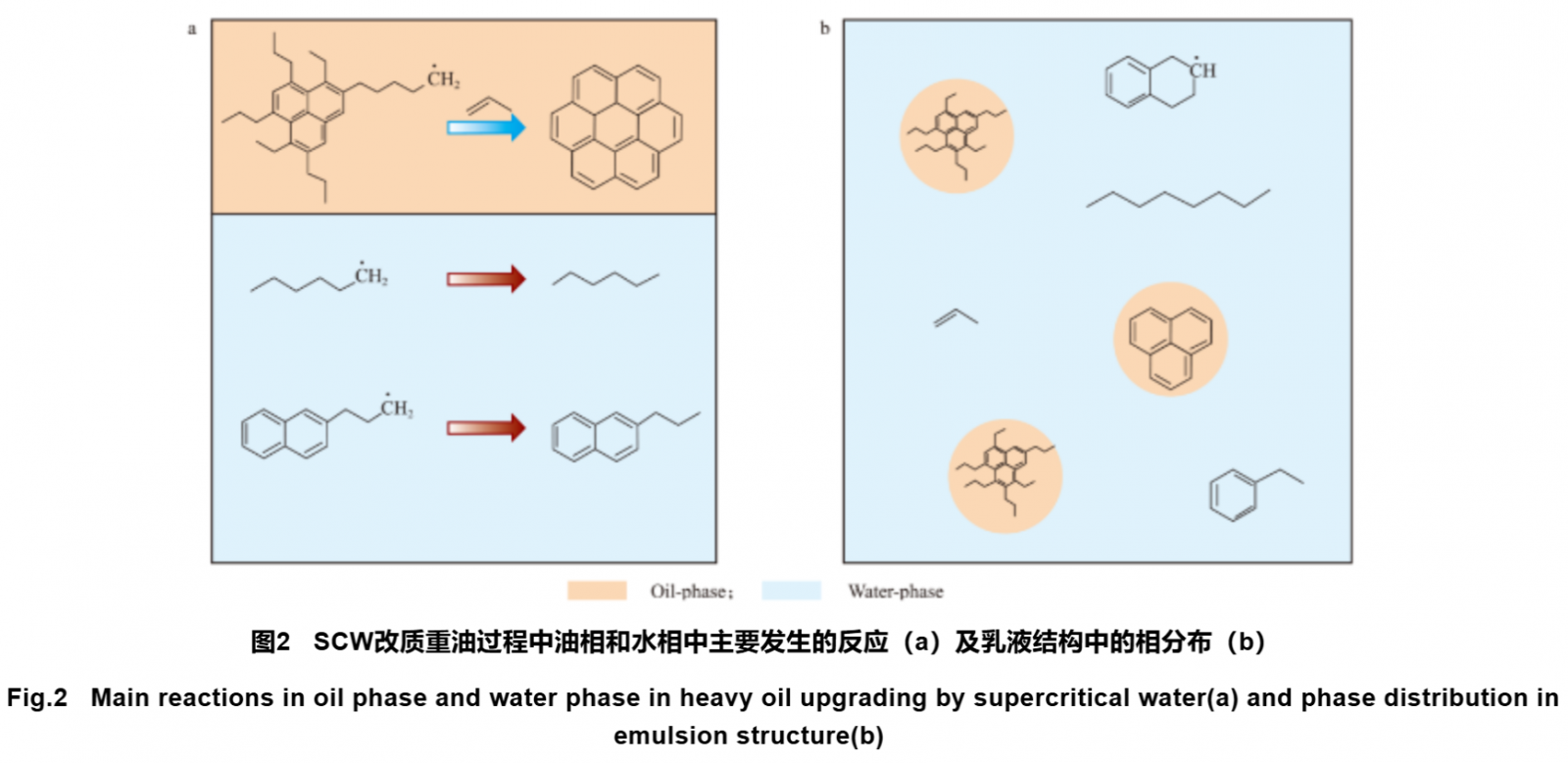
When the water oil mass ratio (water oil ratio) of the SCW modified heavy oil system increases, the system changes from oil and water to lotion structure, as shown in Figure 2b, small droplets formed in the oil phase are evenly distributed in the water phase, and the two phases have a large contact area at this time. In the oil phase, the transfer rate of olefins and methylated aromatic hydrocarbons generated by the cracking of PAHs to the water phase is faster, and the rate of small molecule PAHs generation is lower. The transfer rate of small molecule PAHs to the water phase is also faster. Therefore, the higher the water oil ratio, the better the SCW heavy oil upgrading effect.



