Case Studies
Case Studies
- Construction of A New Organic-inorganic Composite Emulsion and Its Enhanced Mechanical Properties of Oil Well Cement(Part 1)
- Construction of A New Organic-inorganic Composite Emulsion and Its Enhanced Mechanical Properties of Oil Well Cement(Part 2)
- The Application Prospects of DeepSeek Large Model in Petroleum Engineering(Part 1)
- The Application Prospects of DeepSeek Large Model in Petroleum Engineering(Part 2)
- Development and Performance Evaluation of Efficient Asphalt Dispersant (Part 1)
- Development and Performance Evaluation of Efficient Asphalt Dispersant (Part 2)
- Development and Performance Evaluation of Efficient Asphalt Dispersant (Part 3)
- Research and Application Status of Drilling Fluid Plugging Materials (Part 1)
- Research and Application Status of Drilling Fluid Plugging Materials (Part 2)
- Research and Application Status of Drilling Fluid Plugging Materials (Part 3)
2.2 Process Simulation of Polymer Systems
2.2.1 Process Simulation within Polymer Systems
PAM hydrogel is widely used as profile control agent in oil and water wells for profile control and water plugging. Among them, cross-linking agent has an important impact on the gelling performance of PAM hydrogel. The cross-linked PAM represented by water-soluble phenolic forms a cross-linked network structure through covalent bonds. There are many active sites and condensation reaction types in PAM/water soluble phenolic system. The study of crosslinking reaction mechanism is helpful to further develop phenolic crosslinked PAM hydrogels. Ni et al. used density functional theory, MM and MD methods to study the reaction mechanism of water-soluble phenolic aldehyde and PAM crosslinking from the thermodynamic and kinetic aspects of the crosslinking reaction. They further explained the mechanism by combining XPS characterization results and established a network structure of the crosslinked polymer (Figure 3). The average density and number of crosslinking points of the crosslinked polymer were calculated and simulated. The simulation results indicate that the density of the cross-linked product decreases, which is due to the network supported by trimethylolphenol (THMP) between PAM molecules after cross-linking, and the loose arrangement of amorphous polymer molecular chains. In addition, it can be seen from the type of cross-linking points that the number of cross-linking points of PAM/THMP is greater than that of THMP/THMP, and the amide group involved in the cross-linking reaction accounts for about 60% of the total amide group. Further structural characterization of the cross-linked product using XPS shows that the total number of amide groups involved in the reaction accounts for 61% of the total amide group, which is consistent with the calculation and simulation results. It is confirmed that the condensation reaction of PAM and THMP in the system is the main dynamic reaction. Understanding this mechanism will help to develop a new type of PAM gel.
.png)
In addition to organic cross-linked polymer system, inorganic cross-linked polymer gel system is also widely used in low-temperature oil and gas wells. Hamza et al. crosslinked aluminum acetate (AlAc) with PAM, and added bentonite as an additive to improve the stability of the gel system. In this study, the effects of AlAc particle size and the amount of bentonite added on the PAM/AlAc gel process were investigated. The experimental results show that the low content of〔0.5%~1.0% (w)〕bentonite can be used as an additive to delay the crosslinking process of small particle size AlAc, and will not have a significant impact on the strength of gel. Simulate and calculate the relevant parameters of Al3+ adsorption on the surface of bentonite. The adsorption energy of Al3+ is 11.854 eV. When another Al3+ is added to the simulation system, due to the repulsive effect of charges between Al3+, the adsorption energy decreases to 9.25 eV, and the Al-O bond length increases to 0.185 nm. The adsorption of Al3+ on the bentonite surface hindered the interaction between AlAc and PAM, thus prolonging the gel time.
The combination of polymers and surfactants can produce synergistic effects, resulting in excellent performance of the composite system. In recent years, research on the interaction between polymers and surfactant systems has become a topic of interest for researchers, and with the introduction of molecular simulation technology, the study of polymer surfactant systems has gradually deepened from macroscopic properties to microscopic structures. Various ordered aggregates can be formed in a mixed system of polymers/surfactants, such as spherical aggregates, rod-shaped aggregates, vesicles, etc. Hu et al. investigated the self-assembly behavior of a mixture of HPAM and cationic surfactant dodecyltrimethylammonium bromide (DTAB) using CGMD method and Martini force field simulation. The Martini force field was parameterized systematically and is a coarse-grained force field suitable for molecular dynamics simulation of biomolecular systems. This study investigated the effects of HPAM hydrolysis degree and DTAB concentration on self-assembly morphology and formation process at the micro level (Figure 4). Under different hydrolysis conditions, with the increase of DTAB concentration, the aggregates transform from spherical to rod-shaped, and under high hydrolysis conditions, the rod-shaped aggregation structure tends to bend (Figure 5). The effect of DTAB concentration on the transformation of ball/rod morphology was quantitatively analyzed by calculating relative shape anisotropy and Rg. Simulation results showed that the spatial hindrance effect caused by the increase of DTAB concentration had an important impact on the self-assembly morphology of aggregates. Exploring the dynamic information of the three-dimensional structure and self-assembly process of polymer/surfactant mixtures at the molecular level is beneficial for expanding their applications.
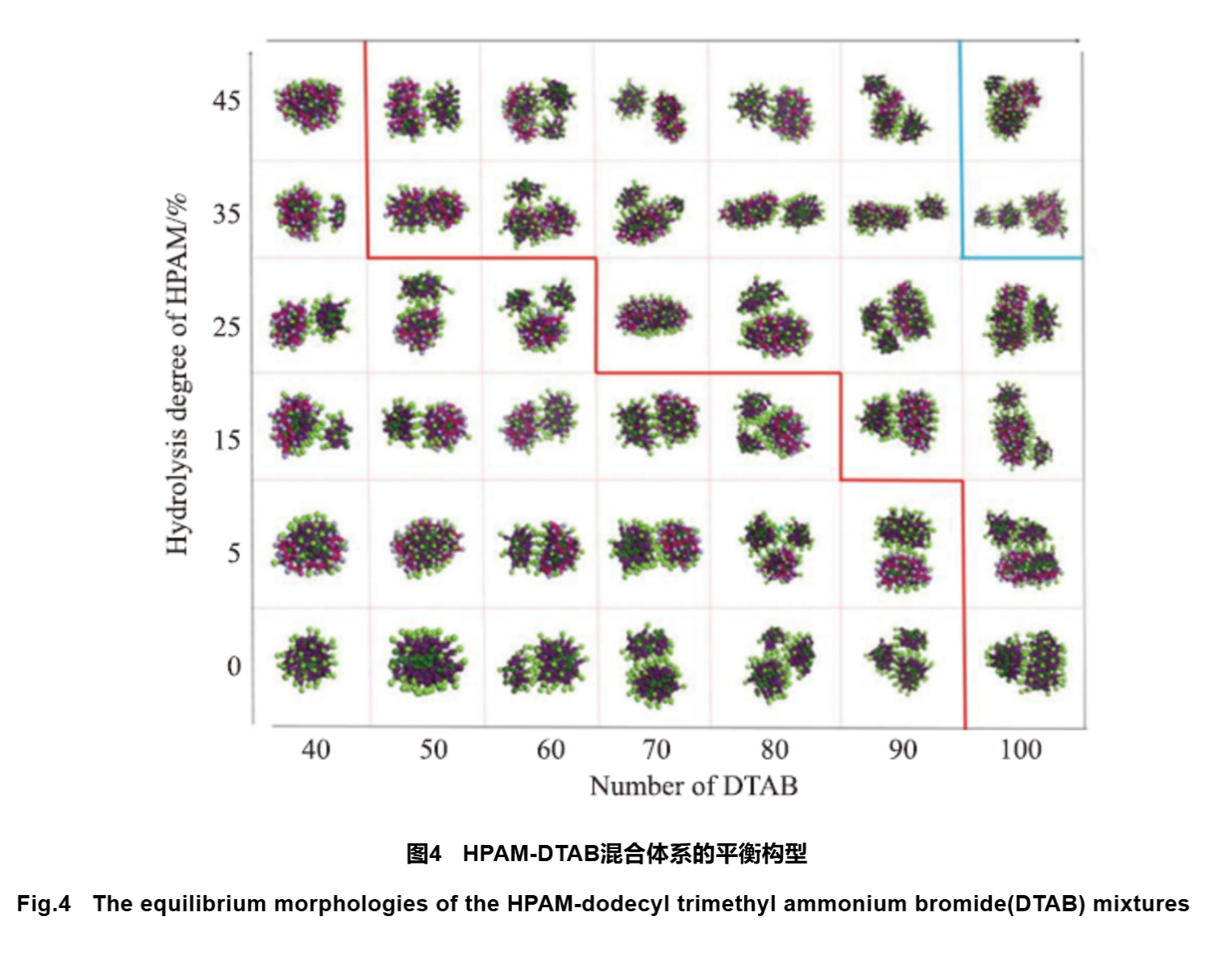
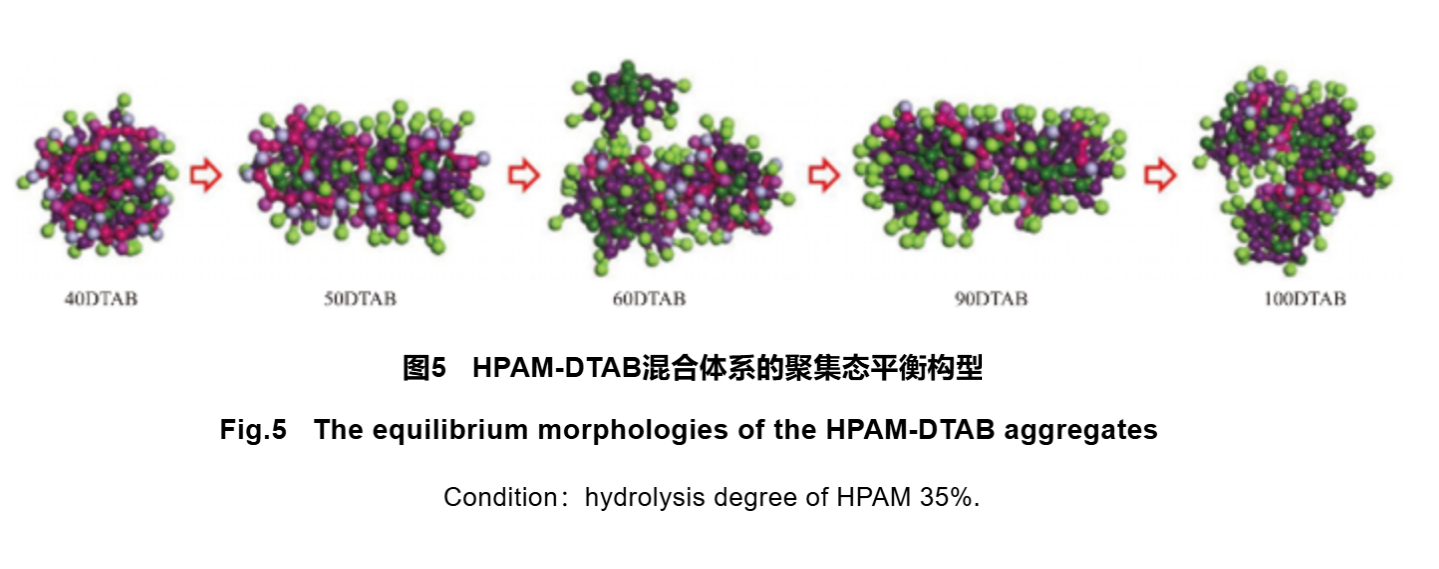
2.2.2 Simulation of Polymer Flooding Process
Polymer solutions have both viscosity and elasticity, and as displacement agents, they must have sufficient ability to overcome the interaction between oil and rock walls. PAM is the most widely used oil displacement polymer, and a deep understanding of the displacement mechanism of polymer oil displacement is of great significance for improving oil recovery. At present, physical simulation experiments such as sand filled pipe models and artificial rock cores have become the main means of studying the mechanism of polymer flooding, but these experiments are still limited to the level of macroscopic oil recovery. More and more researchers are exploring the mechanism of polymer flooding from the perspective of molecular theory. Song Kaoping proposed to describe the molecular forces involved in the polymer flooding process based on the relevant theories of molecular dynamics. By comparing the molecular effects of water flooding, he proved that the viscoelasticity of polymer solutions is a macroscopic reflection of the friction and impact forces between polymer molecules and crude oil molecules, thereby confirming that polymer flooding can significantly improve oil recovery efficiency.
Based on molecular simulation, the study of the micro flow mechanism of polymer flooding at the atomic scale can provide a theoretical basis for improving oil recovery technology in complex oil reservoirs through polymer flooding. Fan et al. investigated the molecular mechanism of using polymer viscoelasticity for oil recovery. A model of viscoelastic polymer displacement of residual oil in nanochannels was established using MD simulation method, and the displacement process of trapped oil droplets in nanochannels was studied (Figure 6). A comparison was made between the oil displacement effects of water flooding and polymer flooding with different chain lengths, and it was found that there were significant differences in the oil displacement effects of polymers with different chain lengths. According to Figure 7, as the chain length increases, using a smaller amount of polymer can displace more oil, resulting in higher micro oil recovery efficiency. The analysis results of polymer viscoelasticity show that with the increase of polymer chain length, the storage modulus increases, the relaxation time prolongs, and the time required for adjusting the configuration of polymer long chains is extended, resulting in an enhanced polymer elastic effect. This enhancement of elasticity allows the polymer to stretch more in the pores and apply stronger pulling force to the oil droplets trapped in dead corners, which is beneficial for improving oil recovery efficiency.
.png)
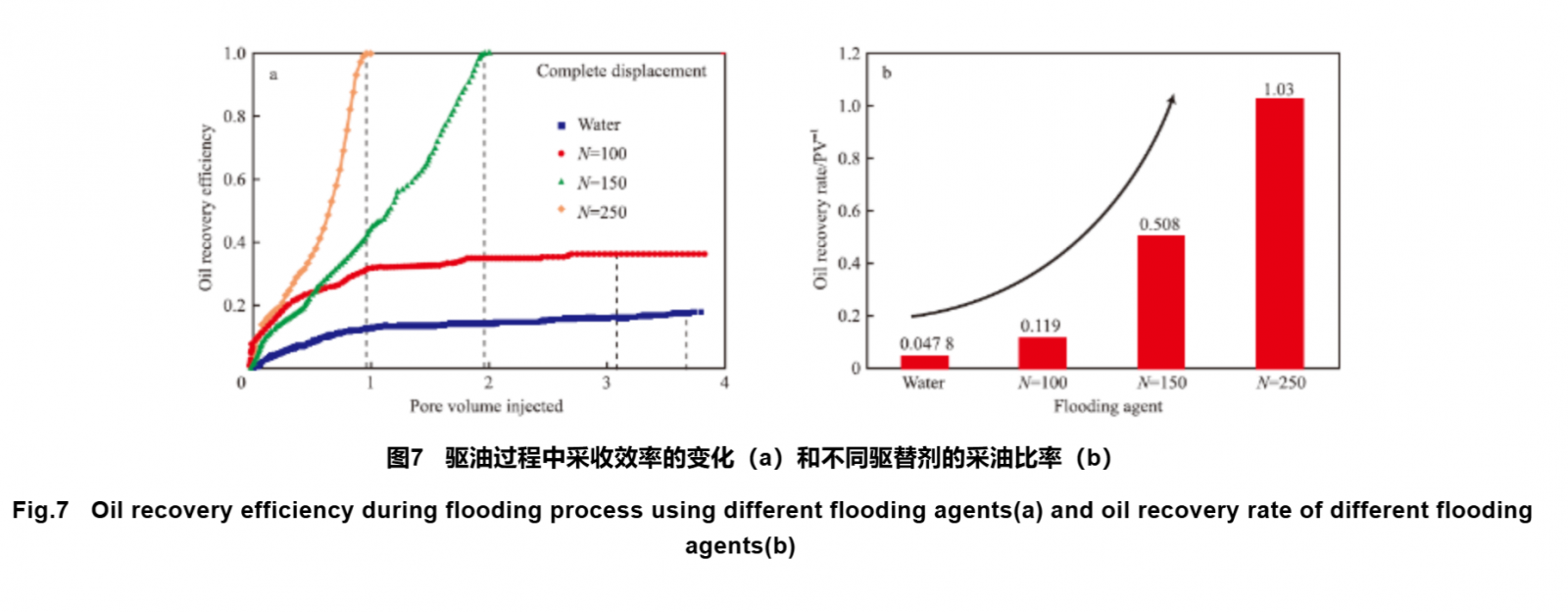
During the oil recovery process, in addition to the influence of viscosity, the wettability of rocks can reflect the affinity or hydrophobicity of the rock surface towards oil and water. If the rock is hydrophilic, water can easily drive oil away from the surface of the rock; If the rock is lipophilic, it is not easy to drive oil away from the surface of the rock. Exploring the effect of polymers on rock wettability can lay the foundation for further developing polymers as wettability regulators in enhanced oil recovery technologies. Ahsani et al. used MD simulation method to conduct molecular dynamic simulation of the adsorption process of HPAM on rock surfaces, and observed the changes in wettability of carbonate reservoirs by HPAM at the microscopic scale. They calculated the non bonding energy, bonding energy, and surface tension of the polymer, water molecules, and rock three-phase system. Under the conditions of environmental temperature and reservoir temperature, when the polymer adsorbs on the surface of carbonate, the adsorption contact angle of the polymer decreases to 130°and 140°, respectively, from high lipophilicity to moderate lipophilicity, that is, the surface wettability tends to shift towards water wettability. This simulation result is consistent with the experimental results. The effect of temperature on the contact angle of HPAM adsorption on rock surface was investigated, and the simulation results were opposite to the experimental results. The simulation results showed that in the Lennard Jones model, as the temperature increased, the molecular kinetic energy increased and the polymer desorbed on the rock surface; Under experimental conditions, due to the occurrence of ion exchange and thermal decarboxylation phenomena, the adsorption of polymers on the rock surface increases with the increase of temperature, and the polymer surface tends to be in a water wet state.
3.Conclusion
PAM has a wide range of applications in the field of oil fields, and molecular simulation technology can be used to deeply study the micro mechanisms of polymer system structure and size changes. At present, the simulation research system mainly studies polymer viscoelasticity, intermolecular interactions, and interactions between molecules and rock walls at the molecular level of PAM, in order to analyze the performance of polymer systems and explain the oil recovery process. With the continuous progress of oilfield development technology, the use of molecular simulation technology to guide experimental research is an inevitable trend in the future, such as the micro mechanism of functional polymers, rational design of polymer molecules, and effect prediction. With the continuous development of computer technology, the system that can be simulated in the future will become more complex, and the simulation results will also be more accurate. Molecular simulation technology will play a more powerful role in the field of oil fields.



